This document discusses drug metabolism, which involves the biochemical alteration of drugs in the body. It covers the major pathways and enzymes involved in drug metabolism.
Drug metabolism occurs primarily in the liver and involves two phases. Phase I reactions modify the drug's structure through oxidation, reduction or hydrolysis. This makes the drug more polar and able to be excreted. Phase II reactions conjugate drugs to endogenous compounds like glucuronic acid, making the drug even more polar and excretable.
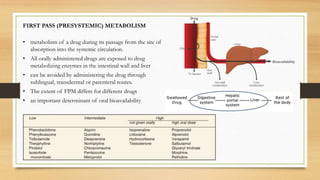
Cytochrome P450 enzymes, especially CYP3A4, are responsible for metabolizing over 50% of drugs. First-pass metabolism occurs when orally administered drugs are metabolized in the intestines and liver before reaching