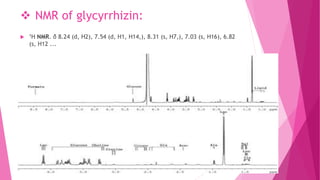
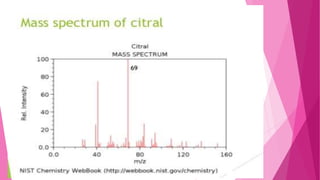
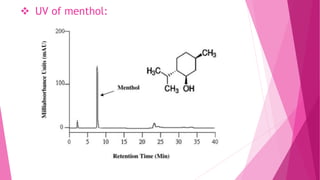
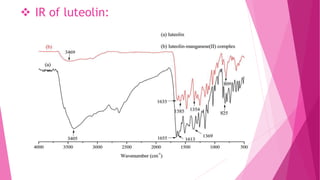
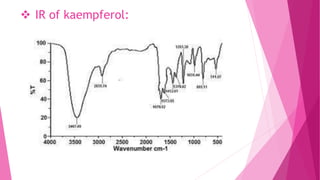
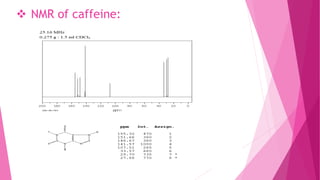
1. The document discusses the structure elucidation of various compounds including carvone, citral, menthol, luteolin, kaempferol, nicotine, caffeine, and glycyrrhizin.
2. Various analytical techniques are described such as UV, IR, mass spectroscopy, and NMR that are used to determine the structure of these compounds.
3. The uses of these compounds are also mentioned including as flavorings, fragrances, medicines, and supplements.














































![ Mass of glycyrrhizin:
Glycyrrhizin; LC-ESI-QTOF; MS2; CE:Ramp 5-60 V; [M+H]+
Molecular Weight: 822.942 g/mol.](https://image.slidesharecdn.com/structureelucidationphytofinal-230914182627-62df352d/85/Structure-elucidation-phyto-pptx-52-320.jpg)