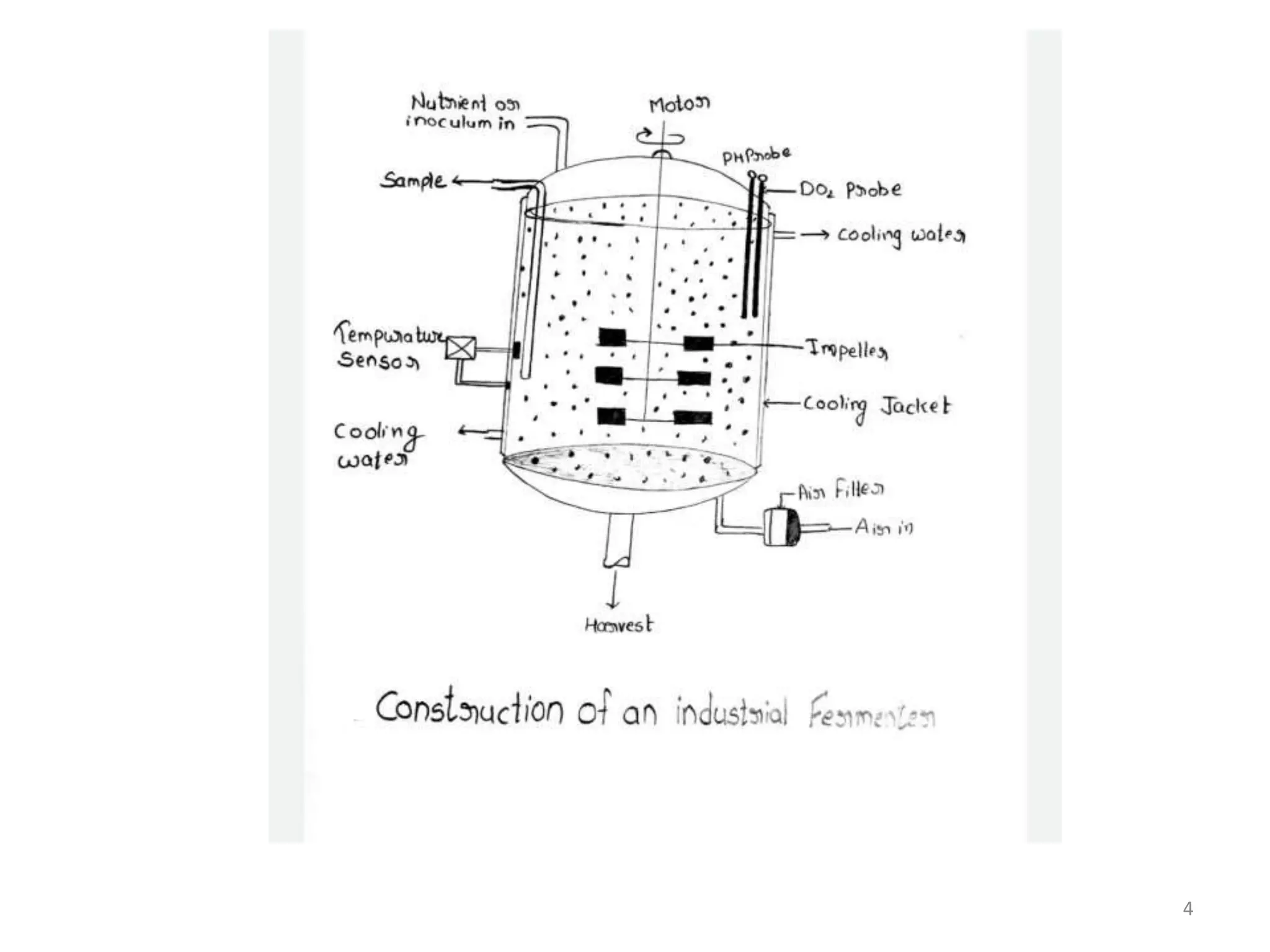
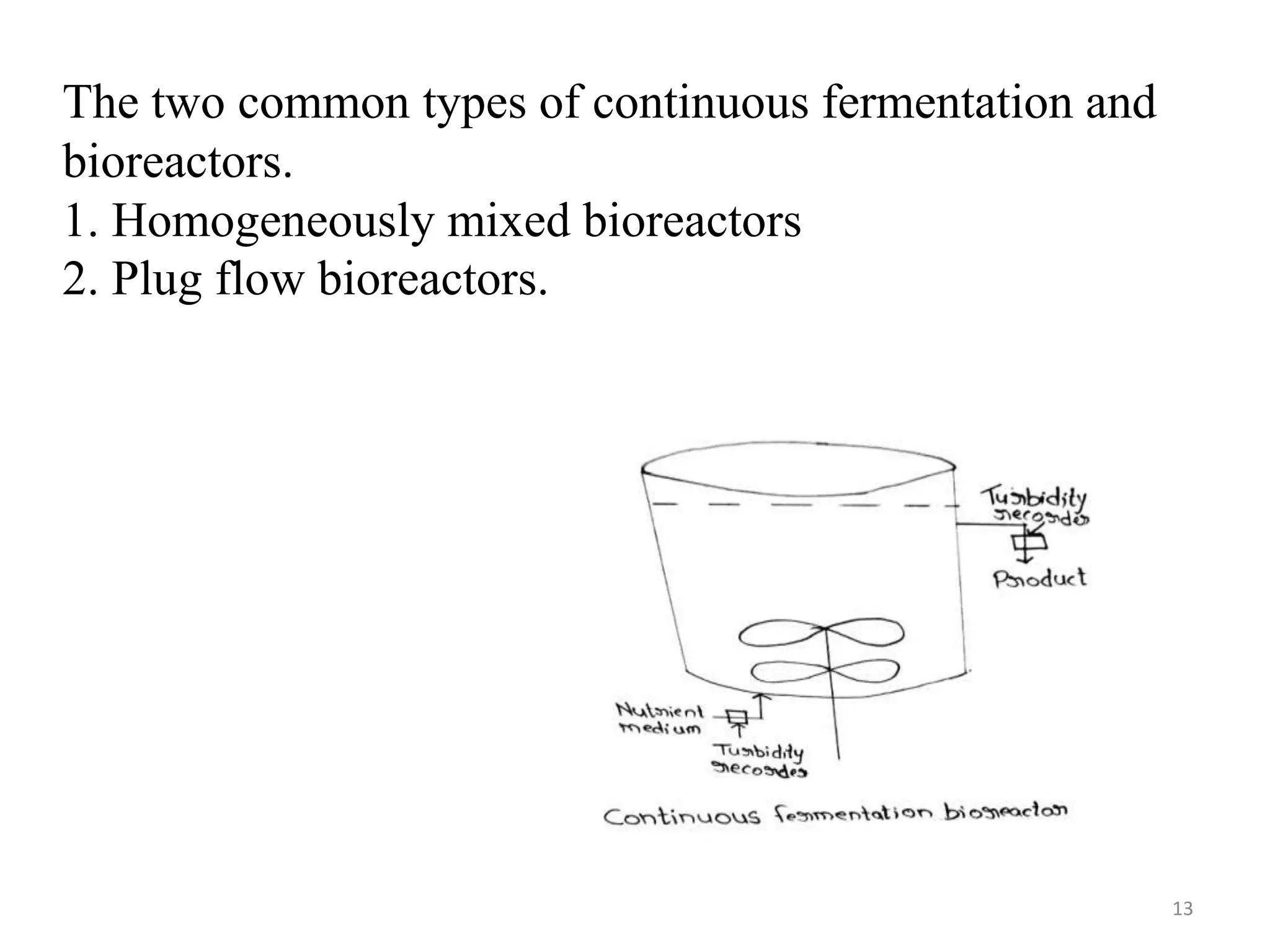
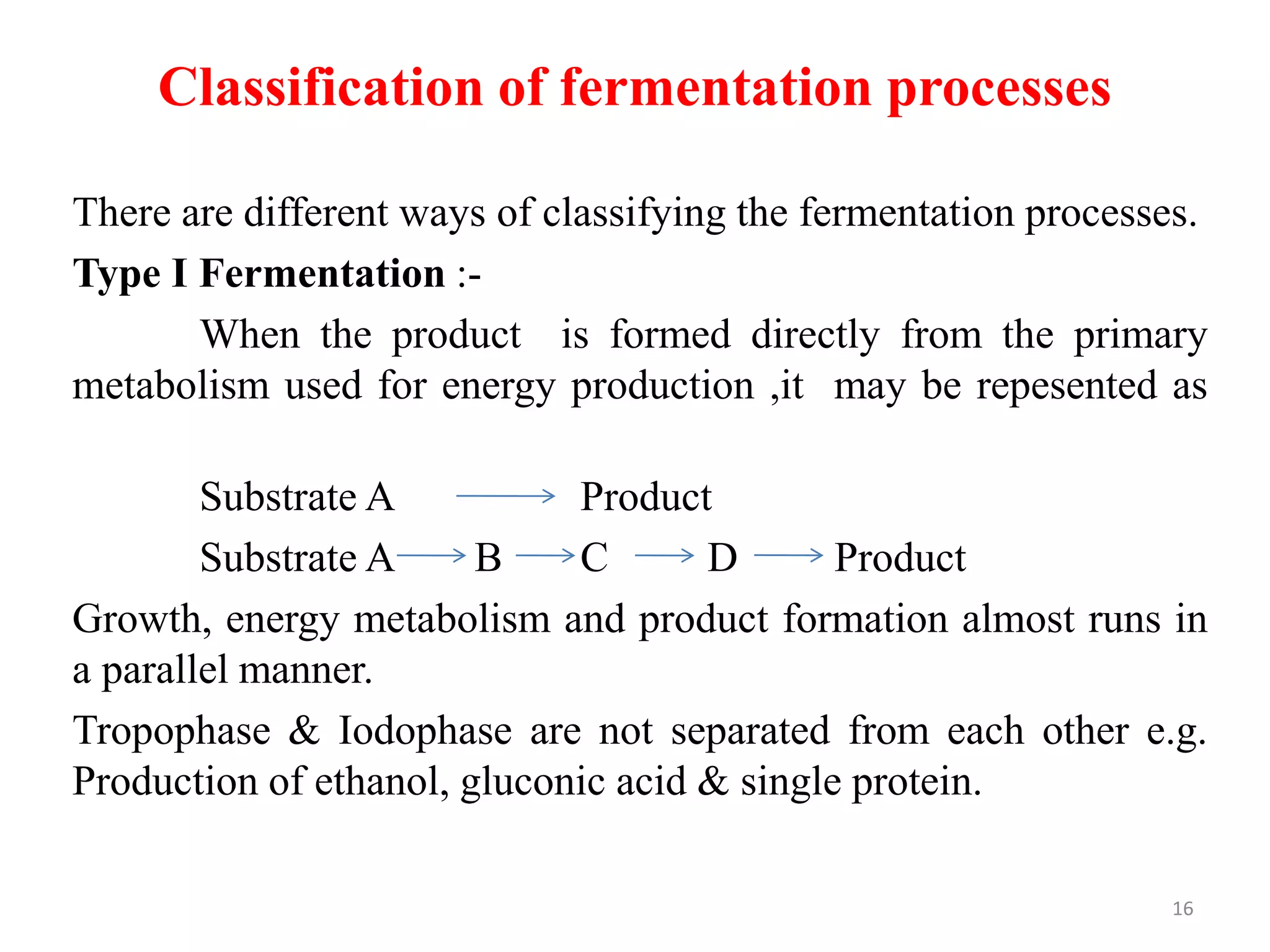
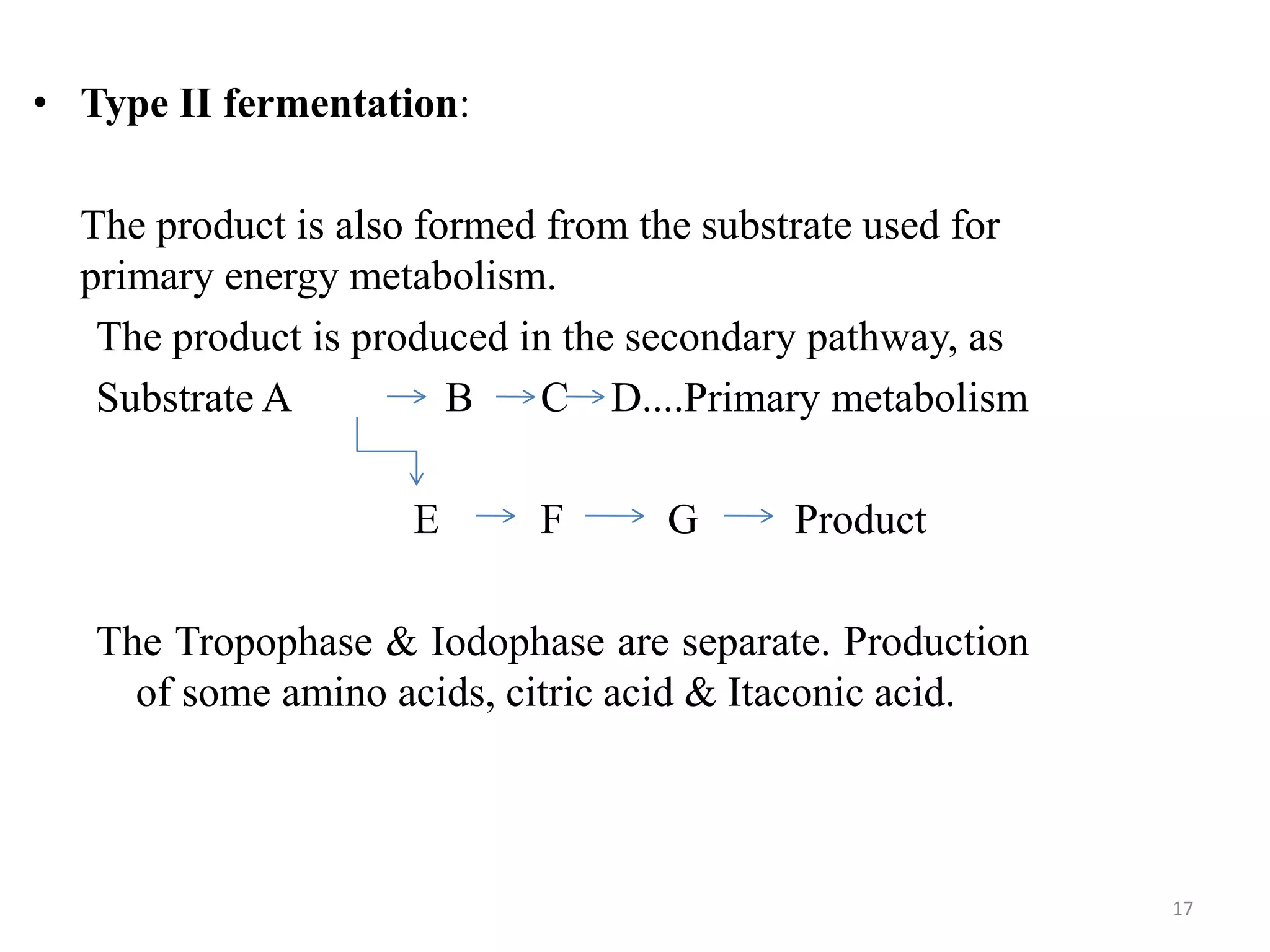
The document provides an overview of fermentation processes, detailing types such as batch and continuous fermentation, and the use of bioreactors for microbial cultivation. It discusses the production of antibiotics like penicillin and streptomycin, as well as vitamins such as riboflavin, highlighting their industrial significance and production conditions. Additionally, the document explains different fermentation classifications, processes, and underlying metabolic pathways involved in biotechnological applications.