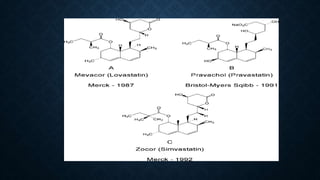
- Statins are drugs that lower cholesterol by inhibiting the enzyme HMG-CoA reductase. Some early statins like lovastatin are produced naturally by fungi through fermentation.
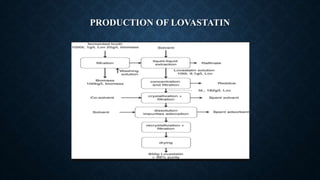
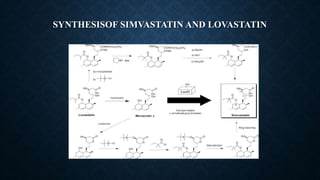
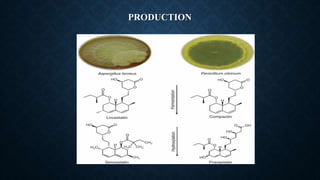
- Lovastatin is produced commercially by fermenting the fungus Aspergillus terreus. Simvastatin is then produced from lovastatin through a chemical reaction.
- Solid state fermentation and submerged fermentation techniques can be used to produce statins at an industrial scale. Optimization of fermentation parameters and media composition aims to improve statin yields.