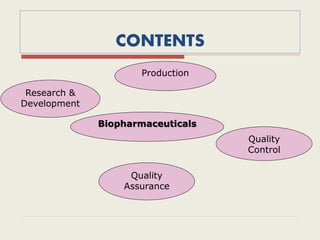
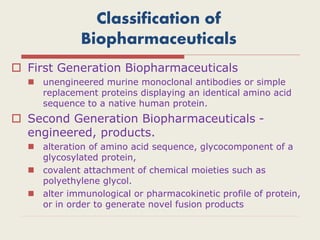
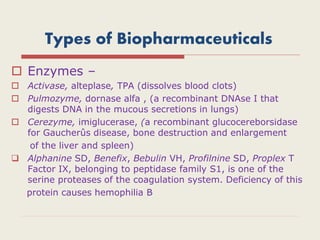
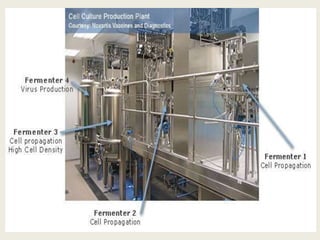
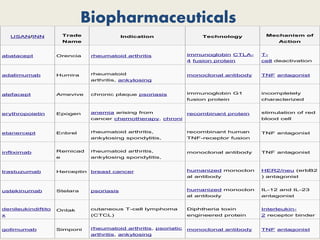
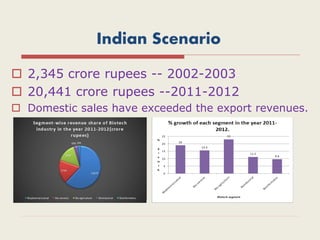
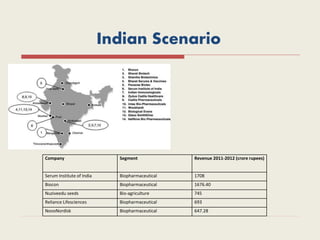
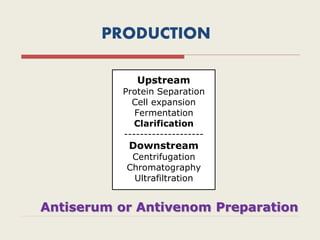
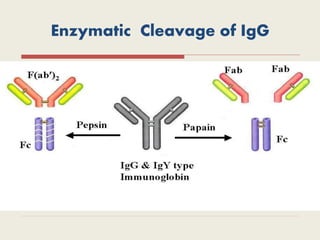
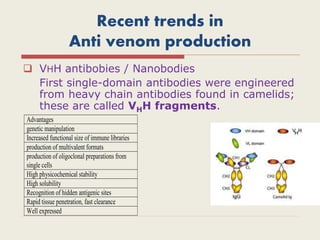
The document provides an overview of biopharmaceuticals. It discusses that biopharmaceuticals are biologically significant compounds derived from living cells that are used to treat various human health disorders. It then covers classification of biopharmaceuticals, examples of different types including antibodies, cytokines, hormones, enzymes, vaccines, and monoclonal antibodies. The document also discusses production processes, quality control, and future trends in the biopharmaceutical industry.