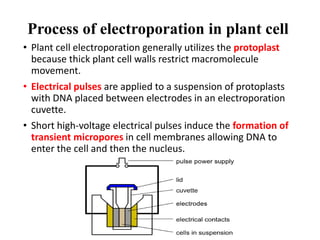
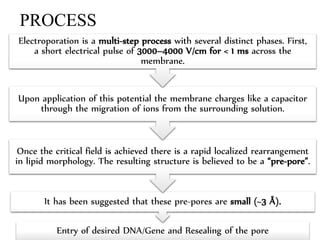
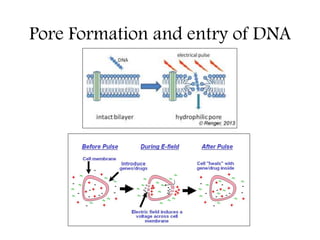
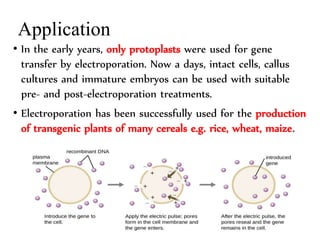
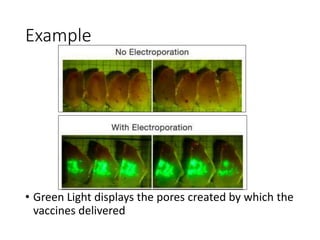
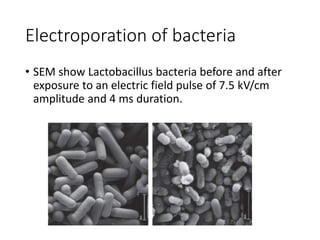
Electroporation is a method to transform cells by creating transient pores in the cell membrane through applying brief high-voltage electric pulses, allowing DNA to enter the cell. It involves suspending cells in a solution with DNA between electrodes and applying pulses of 4000-8000 V/cm for milliseconds. This forms pores in the membrane through which DNA can enter. It is commonly used to transform bacteria, yeast, plant protoplasts, and transfect eukaryotic cells. Key factors influencing electroporation include field strength, pulse length, DNA purity and concentration, and cell growth conditions.