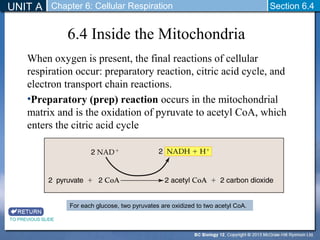
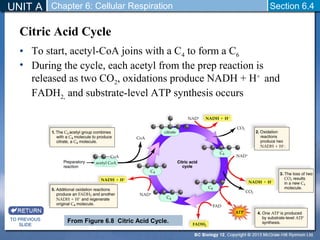
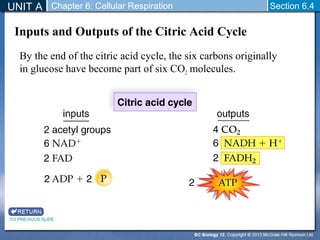
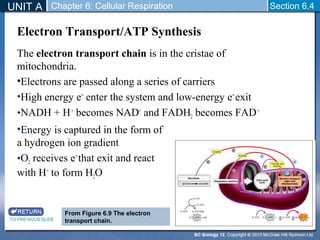
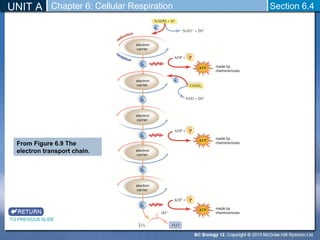
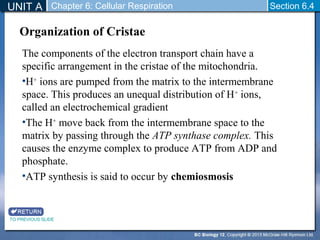
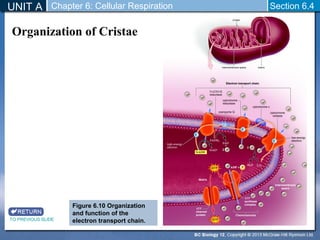
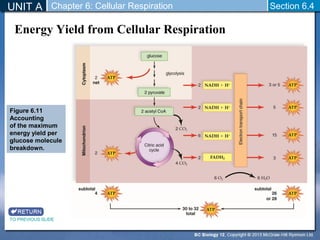
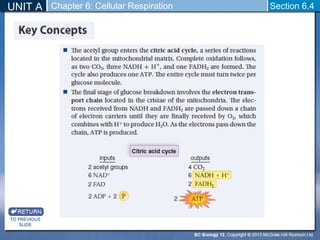
The document outlines the key stages of cellular respiration within mitochondria when oxygen is present. It discusses how pyruvate is oxidized to acetyl CoA which enters the citric acid cycle in the mitochondrial matrix. The citric acid cycle converts the acetyl groups to CO2 while producing ATP, NADH, and FADH2. The electron transport chain uses NADH and FADH2 to establish a hydrogen ion gradient across the inner mitochondrial membrane, allowing ATP synthase to produce ATP through chemiosmosis. The maximum ATP yield from completely oxidizing one glucose molecule is approximately 30 ATP.