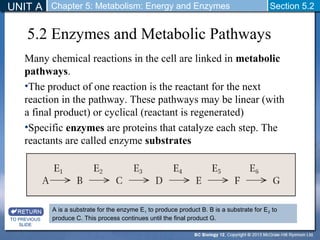
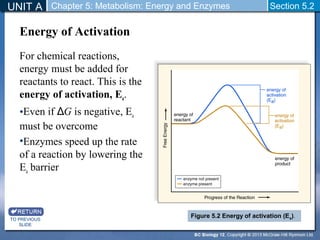
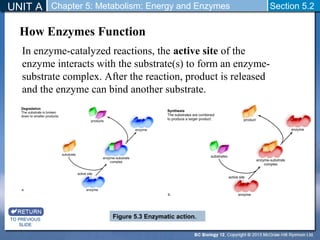
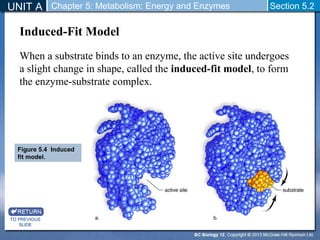
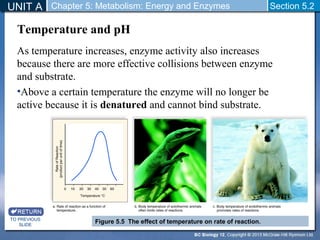
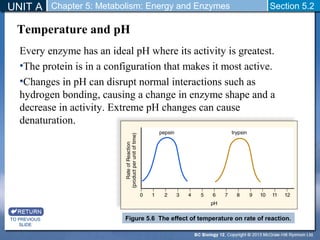
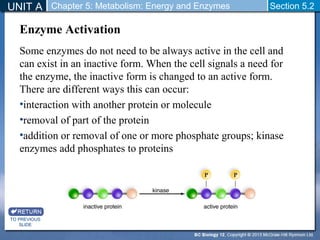
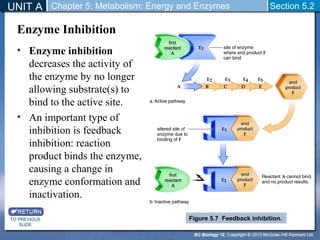
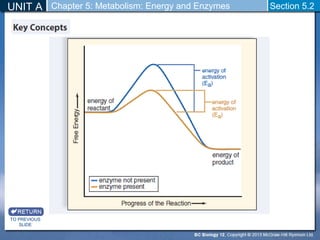
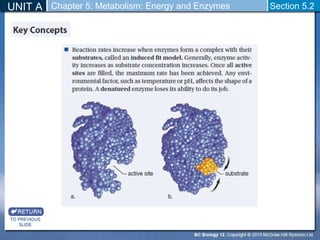
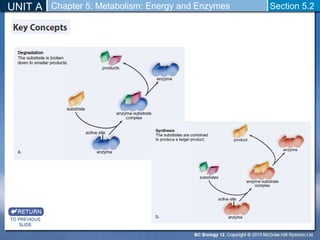
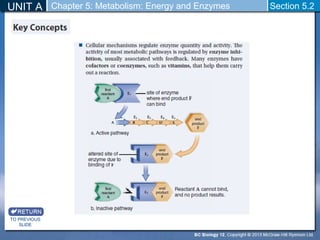
This document outlines a unit and chapter on metabolism, energy, and enzymes. It discusses how enzymes catalyze reactions in metabolic pathways and allows cells to regulate pathways. Enzymes lower activation energy and form complexes with substrates. An enzyme's shape is important for its activity, and factors like temperature, pH, substrate concentration, and inhibitors can impact the rate of reactions. The chapter explores how enzymes are named, activated, regulated through feedback inhibition, and require cofactors to function.