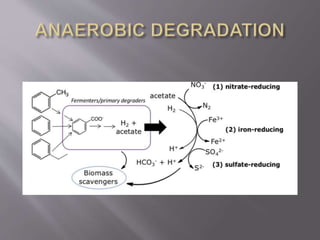
This document discusses hydrocarbon bioremediation. It defines hydrocarbons and explains that they are readily degraded by microorganisms under aerobic conditions. Both bacteria and fungi can aerobically degrade alkenes, alkanes, and aromatic hydrocarbons through different metabolic pathways. While aerobic degradation is faster, some microbes can also anaerobically degrade hydrocarbons through pathways like fumarate addition, oxygen-independent hydroxylation, and carboxylation. The document concludes that bioremediation removes hydrocarbons that are environmental pollutants and contribute to health and climate issues.