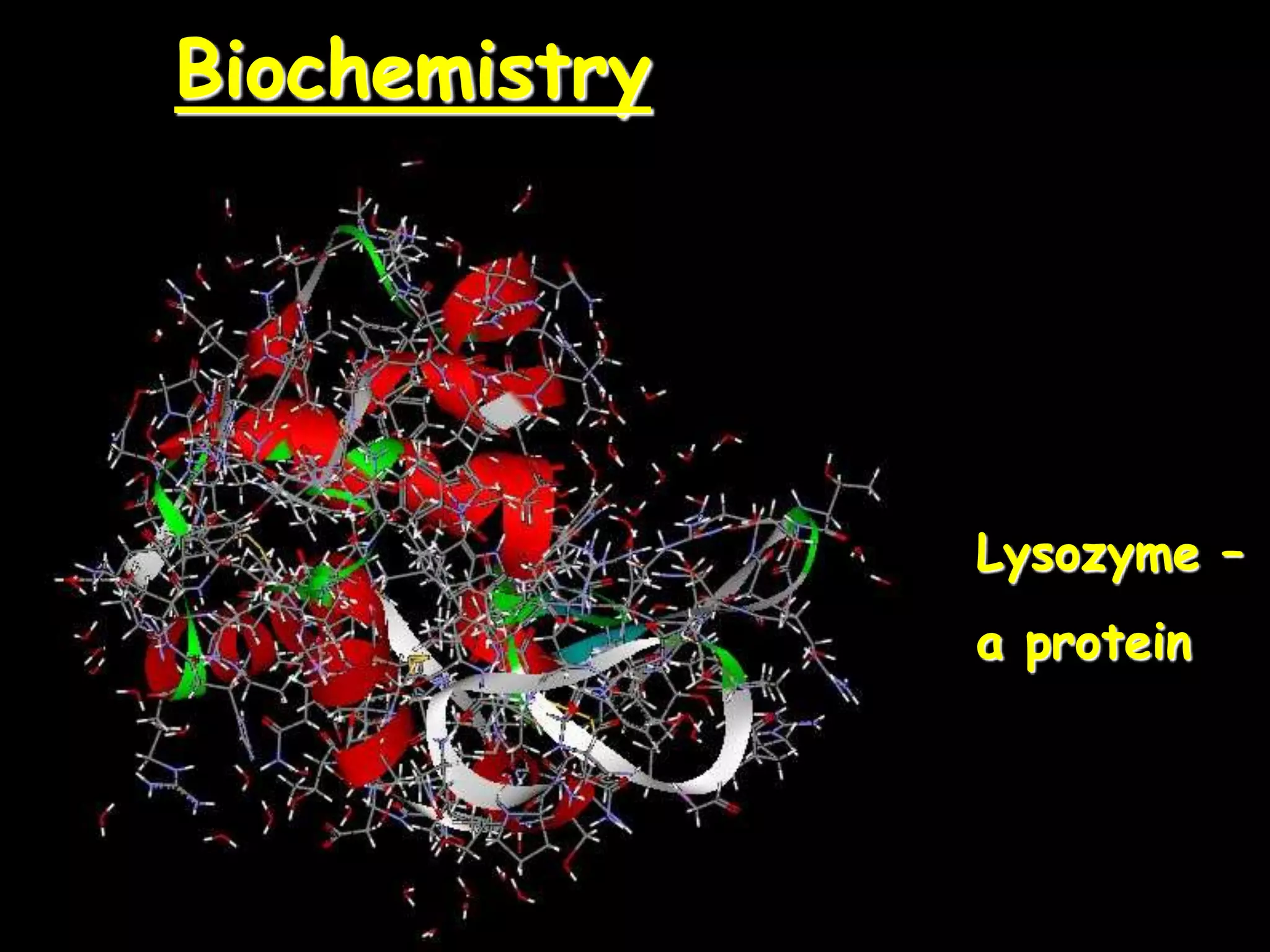
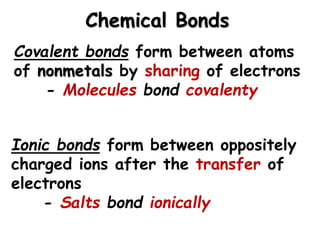
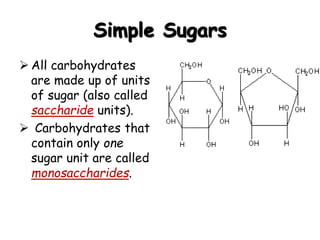
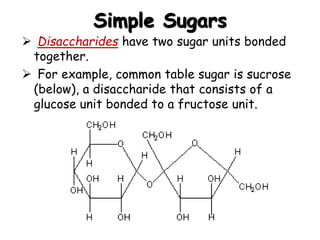
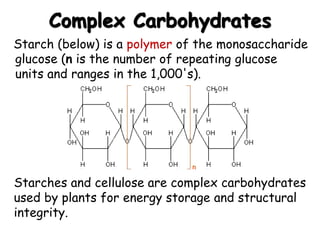
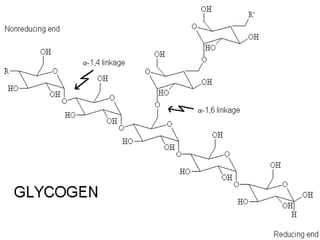
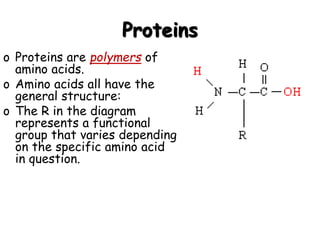
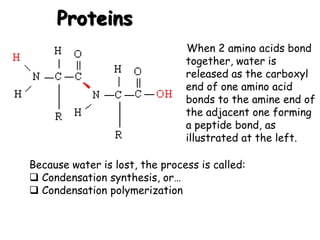
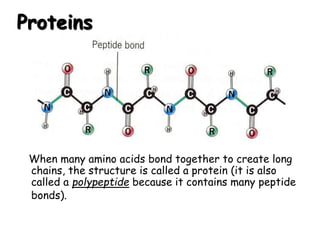
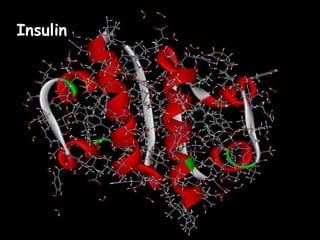
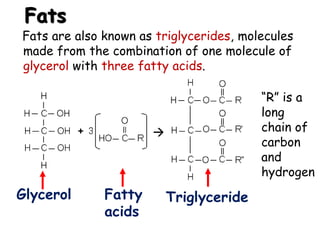
This document provides an overview of key biomolecules including carbohydrates, proteins, and fats. It explains that carbohydrates can be simple sugars like glucose or complex chains known as polysaccharides. Proteins are polymers of amino acids joined by peptide bonds. Fats, also called triglycerides, are made of glycerol bonded to three fatty acids and serve as concentrated energy stores. These biomolecules are essential building blocks of living organisms.