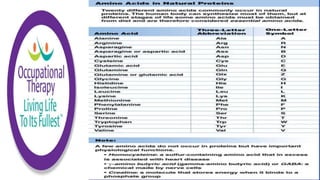
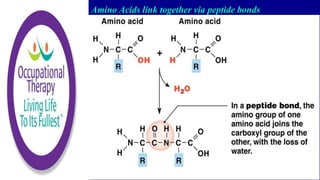
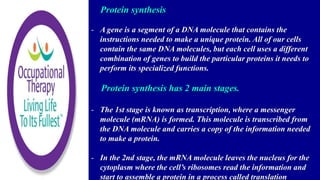
Amino acids are small molecules that are used to produce energy and synthesize other molecules like hormones. They link together via peptide bonds to form polymers called proteins. There are 20 common amino acids that can combine to form proteins. Proteins fold into specific shapes determined by their amino acid sequence and perform important functions like catalyzing reactions, providing structure, transporting molecules, and acting as antibodies. Protein synthesis occurs through transcription and translation - genes provide instructions to form messenger RNA, which then directs ribosomes to assemble amino acids into proteins according to base sequences. Different types of proteins perform roles like enzyme catalysis, immune response, muscle movement, structure, hormone activity, and transport.