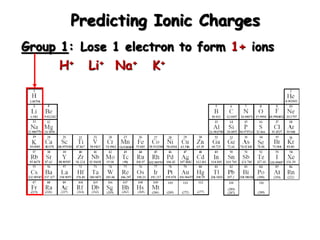
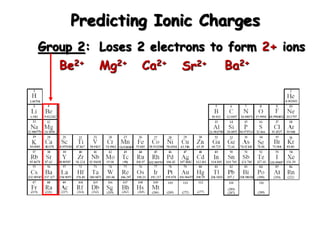
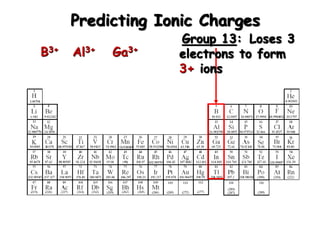
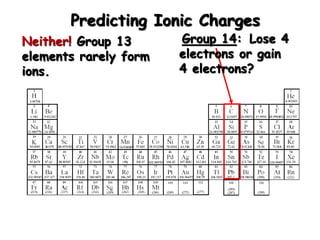
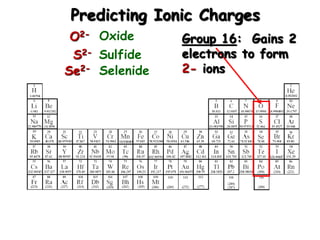
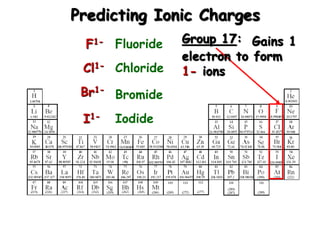
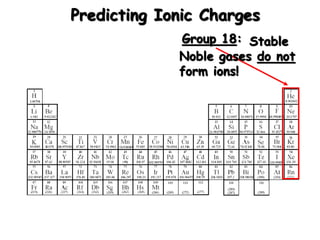
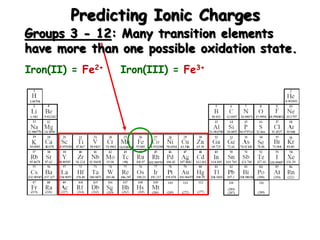
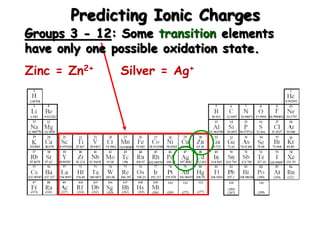
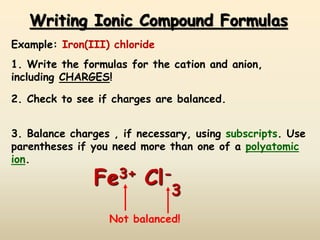
This document discusses ionic compounds, including how to predict ionic charges based on an element's position in the periodic table, write formulas for ionic compounds by balancing charges, and name ionic compounds. Cations are positive ions and anions are negative ions. Groups 1 and 2 metals form 1+ and 2+ ions, respectively, while nonmetals in Groups 13-17 commonly form ions with charges matching their group number. Transition metals can have multiple possible charges. Ionic compound formulas are written by combining cation and anion formulas and balancing their charges with subscripts. Ionic compounds are named by writing the cation name followed by the anion name.