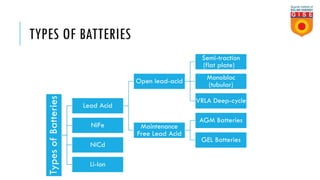
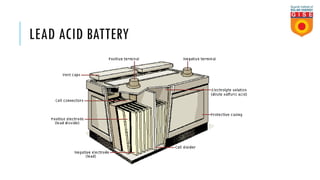
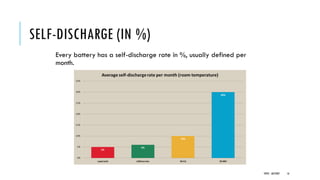
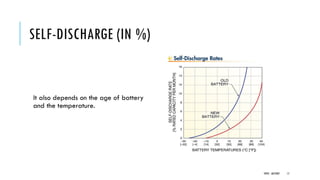
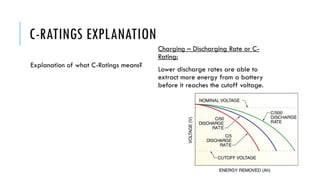
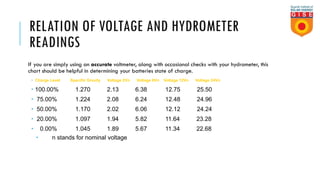
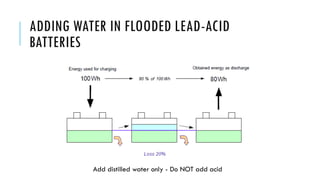
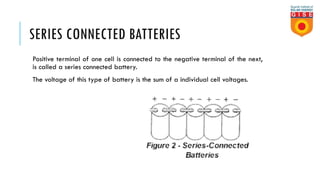
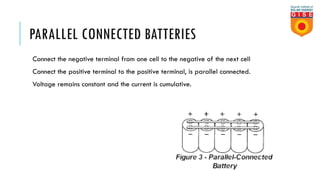
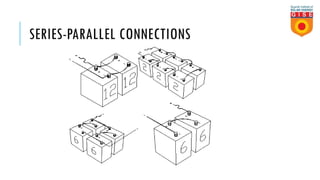
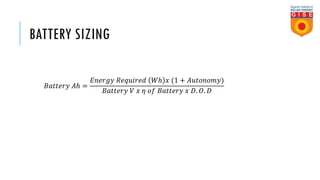
Batteries convert chemical energy into electrical energy through reversible chemical reactions. There are two main types - primary batteries that cannot be recharged and secondary batteries that can be recharged. Lead-acid batteries are commonly used for storage in photovoltaic systems due to their low cost and long life, though they require regular maintenance. Proper ventilation is needed when charging batteries to prevent accumulation of explosive hydrogen gas. State of charge, depth of discharge, and other factors must be considered when selecting and sizing batteries.