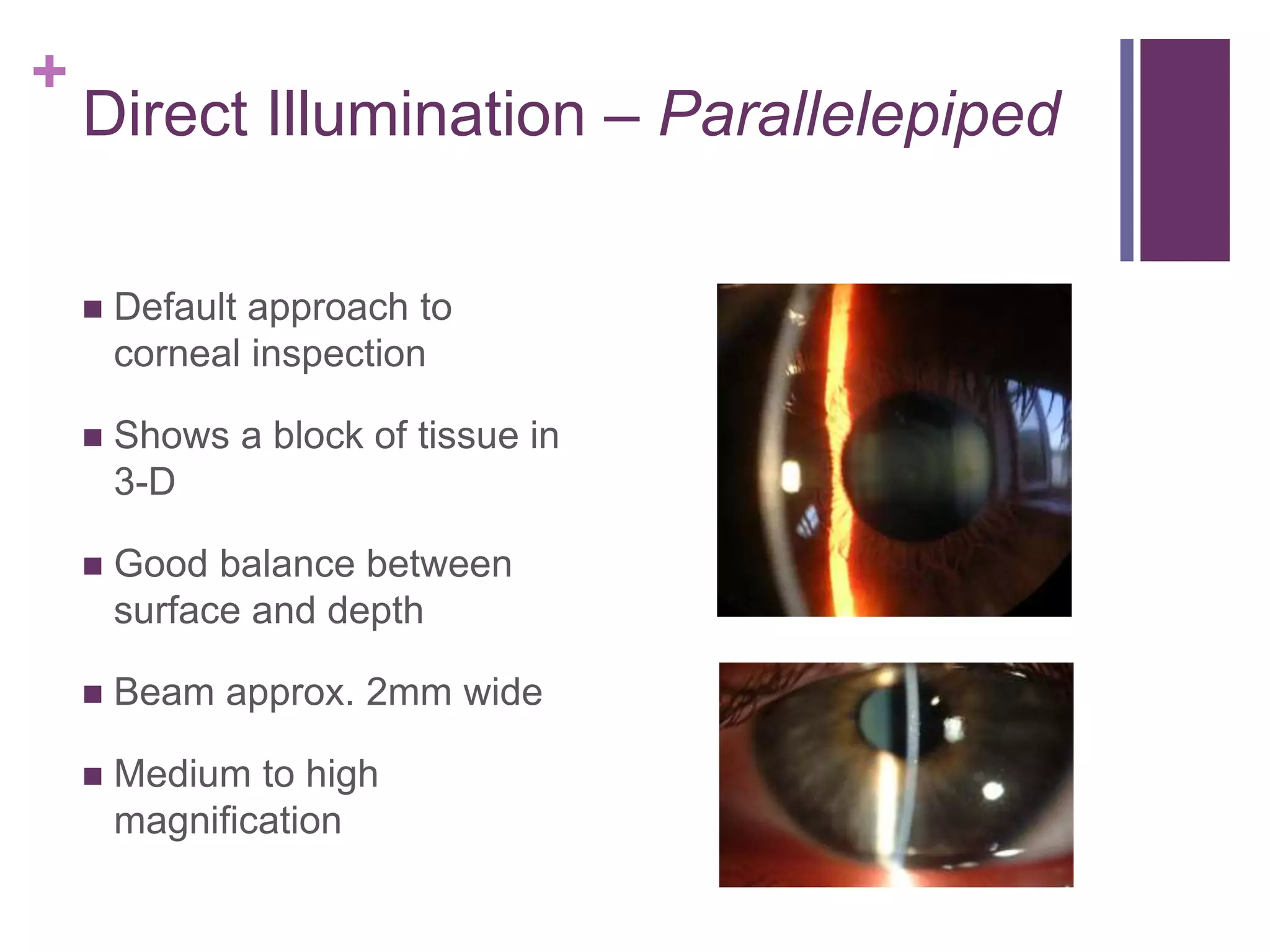
The document provides an overview of slit lamp biomicroscopy. It describes the basic design of the slit lamp microscope including the light tower, viewing arm, and central pivot. It discusses the slit lamp features of magnification, illumination, and examination techniques using direct and indirect lighting. The document outlines the proper procedure for patient setup and examination sequence, including lid eversion and removal of superficial ocular foreign bodies.