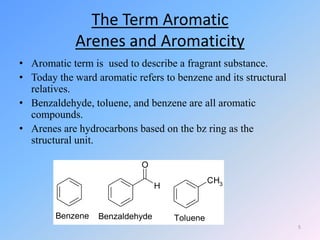
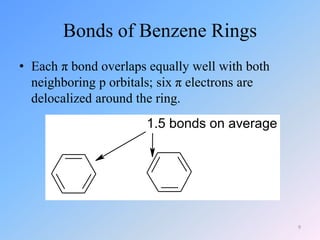
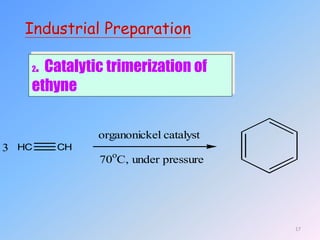
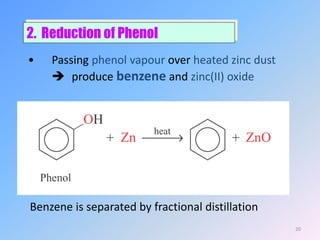
This document discusses organic chemistry (II) and aromatic hydrocarbons. It provides details about the instructor, Dr. Ehssan Nassef, and their office hours. The document then covers various topics related to aromatic hydrocarbons including their classification and properties. Key aspects of benzene such as its structure, stability, preparation methods, and the Huckel rule are explained.