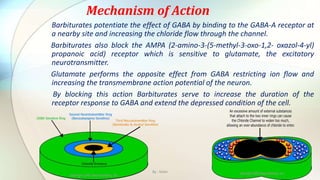
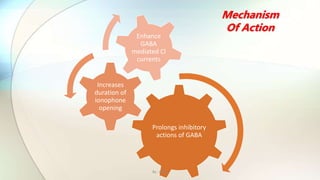
1) Barbiturates are central nervous system depressants that act as sedatives, hypnotics, and anticonvulsants by enhancing the effects of the inhibitory neurotransmitter GABA at GABA-A receptors.
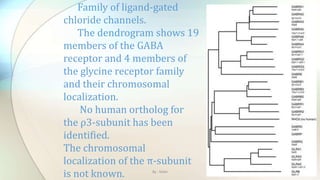
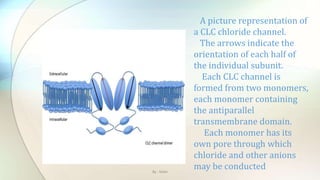
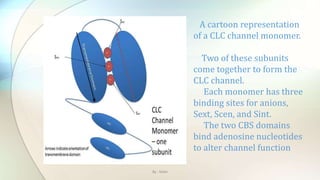
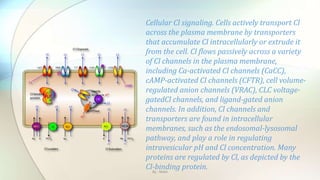
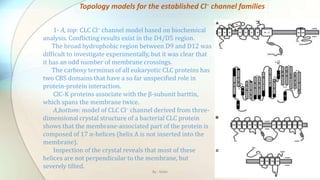
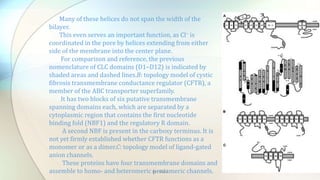
2) Chloride channels are a diverse family of ion channels that transport chloride ions across cell membranes. They play important roles in processes like neuronal excitability, muscle function, and transepithelial salt transport.
3) The document discusses the mechanisms of action and adverse effects of barbiturates, summarizes research on chloride channel families including CLC channels, and references additional sources for more information.










![Chloride channels are a superfamily of poorly
understood ion channels specific for chloride. These
channels may conduct many different ions, but are
named for chloride because its concentration in vivo is
much higher than other anions.[1] Several families
of voltage-gated channels and ligand-gated channels
By : Mahi](https://image.slidesharecdn.com/kazanstatemedicaluniversity-161229152254/85/Barbiturates-and-Chloride-channel-13-320.jpg)
![Transepithelial transport models. A: potassium secretion in the stria vascularis of the cochlea needs basolateral
Cl−channels for recycling Cl− that is transported into the cell by a Na+-K+-2Cl− cotransporter (NKCC1). K+ is secreted apically
via KCNQ1/KCNE1 potassium channels. The basolateral membrane most likely contains parallel heteromeric ClC-
Ka/barttin and ClC-Kb/barttin Cl− channels (147). Mutations in KCNQ1, KCNE1, NKCC1, and BSND (encoding barttin)
cause deafness, but mutations in either ClC-Ka or ClC-Kb alone do not. B: chloride reabsorption in the thick ascending limb
of Henle's loop involves an apical Na+-K+-2Cl− cotransporter (NKCC2) that needs a parallel K+ channel (ROMK1, Kir1.1) for
recycling potassium. Cl− leaves the cell passively across the basolateral membrane through the ClC-Kb/barttin Cl−channel
(147). Mutations in NKCC2, ROMK, or ClC-Kb cause variants of the same disorder, Bartter's syndrome. Mutations in the β-
subunit barttin (BSND) cause Bartter syndrome with deafness, as its loss of function affects both ClC-Ka and ClC-Kb.C:
chloride secretion in intestinal crypt cells. Intracellular Cl− concentration ([Cl−]i) is raised above equilibrium by a Na+-K+-
2Cl− cotransporter that needs a parallel K+ channel (KCNQ1/KCNE3) for recycling and passively leaves the cell via the
apical cAMP-stimulated Cl−channel CFTR. By : Mahi](https://image.slidesharecdn.com/kazanstatemedicaluniversity-161229152254/85/Barbiturates-and-Chloride-channel-19-320.jpg)



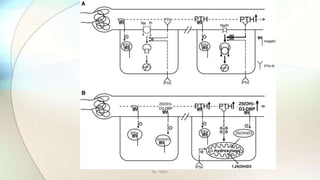
![• Proximal tubular defect in endocytosis leads to secondary changes in calciotropic hormone levels and to
phosphaturia in ClC-5 KO mice.
• A: mechanism leading to phosphaturia. Parathyroid hormone (PTH) is filtered into the primary urine across the
glomerular filter (left). It can bind to megalin (symbolized by the zig-zag sign), which leads to its internalization
and degradation in lysosomes.
• The reduced endocytosis (symbolized by hyphens) leads to an increased concentration of PTH in later parts of the
proximal tubule compared with wild-type mice. This leads to an increased binding to apical PTH receptors (Y),
stimulating the endocytosis of apical Na+-Pi cotransporters and their degradation in lysosomes.
• This leads to the phosphaturia observed inClcn5 − mice and in human patients with Dent's disease. B: mechanism
leading to changes in vitamin D metabolism. As shown in A, the defect in endocytosis entails a luminal increase in
PTH concentration, resulting in enhanced PTH signaling.
• This increases the transcription of α-hydroxylase, a mitochondrial enzyme that converts 25-hydroxyvitamin
D3[25(OH)D3] to the active hormone 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. On the other hand, the precursor
25(OH)D3, bound to its binding protein, is filtered into the primary urine and is normally endocytosed via megalin.
• This constitutes the main supply of 25(OH)D3 for the α-hydroxylase, reducing the availability of the substrate in
the knockout. The supply of 25(OH)D3 is further compromised by a severe loss of this precursor into the urine
that may lead to decreased serum level.
• Thus the impaired endocytosis leads to two opposing effects on the synthesis of 1,25(OH)2D3: a decrease in the
precursor and an increase in enzymatic activity.
• The relative strengths of these effects determine whether there will be an increase or decrease in the serum
concentration of the active hormone. An increase will lead to increased intestinal Ca2+ reabsorption and,
secondarily, increased renal Ca2+ secretion, eventually causing kidney stones.
By : Mahi](https://image.slidesharecdn.com/kazanstatemedicaluniversity-161229152254/85/Barbiturates-and-Chloride-channel-24-320.jpg)