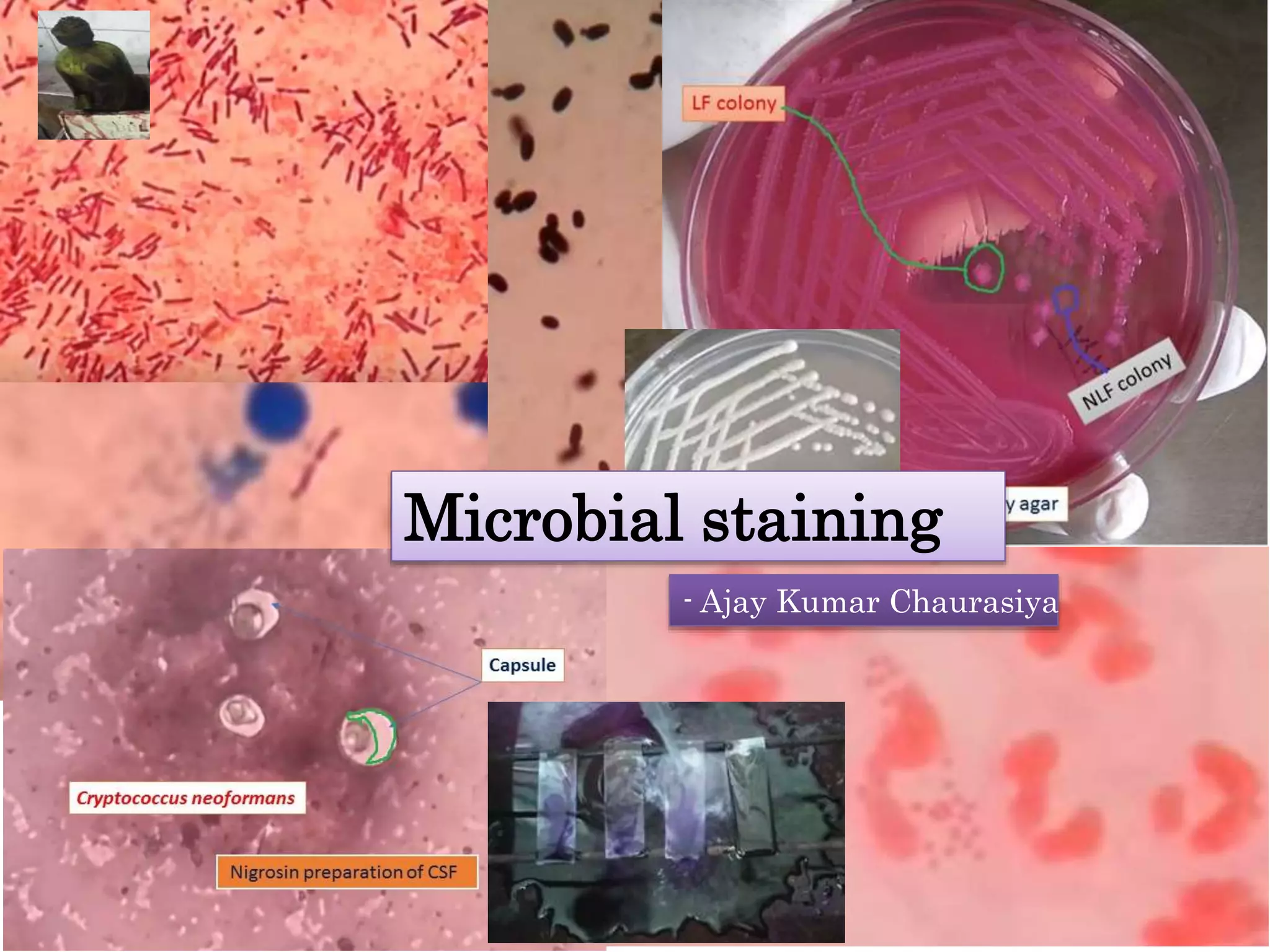
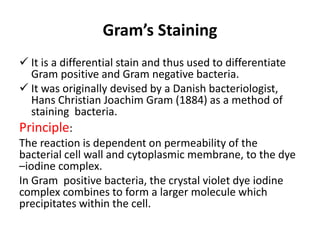
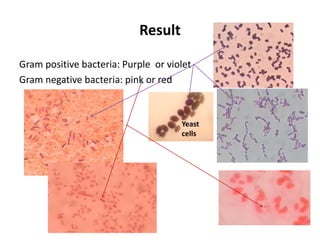
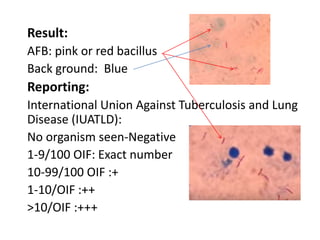
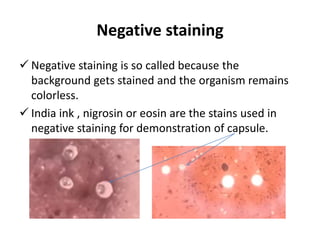
This document discusses various staining techniques used in microbiology to visualize microorganisms under the microscope. It describes simple staining, Gram staining, acid-fast staining, and other specialized staining methods. For each technique, it provides details on the principle, requirements, specimen preparation, procedure, and expected results. The staining methods discussed can be used to differentiate between bacteria and enhance contrast for microscopic examination.