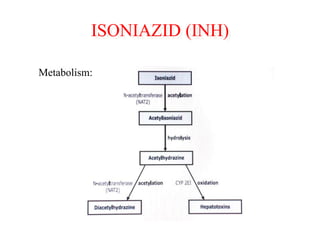
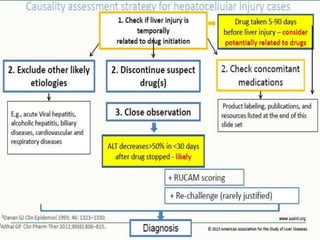
1) ATT induced hepatitis refers to drug-induced liver injury caused by anti-tuberculosis treatment medications like isoniazid, rifampin, and pyrazinamide.
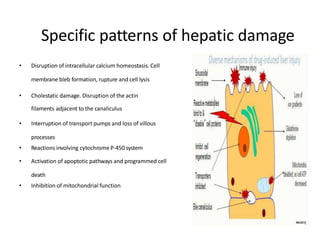
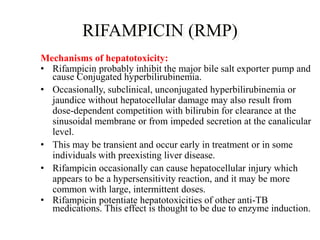
2) These drugs can cause a spectrum of liver damage from asymptomatic transaminase elevations to acute liver failure via both idiosyncratic and dose-dependent mechanisms including intracellular calcium disruption and apoptosis.
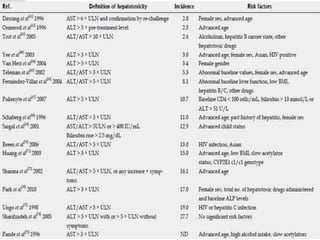
3) Risk factors for tuberculosis drug-induced liver injury include older age, female sex, extra-pulmonary or meningeal tuberculosis, malnutrition, alcohol use, viral hepatitis coinfection, and certain genetic factors. Careful monitoring of liver enzymes is recommended during treatment.

![Definition
• In the absence of symptoms, elevation of
transaminases up to 5 times the upper limit of
normal (ULN) and in the presence of
symptoms up to three times the ULN or twice
the ULN of bilirubin
• Risk of TB DILI ranges from 5 to as high as
33%. [ATS 2006]](https://image.slidesharecdn.com/attinducedhepatitis-190401182334/85/Att-induced-hepatitis-pptx-new-2-320.jpg)
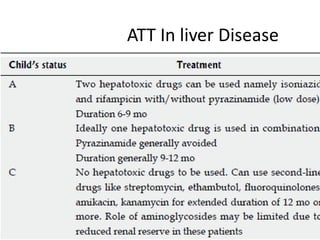
![• Definition of hepatotoxicity in patients with
previous liver diseases is controversial
• Schenker et al reported that elevations in the
ALT and/or AST levels to 50-100 IU/L more
than the baseline levels might define toxicity
]
DILI in LIVER DISEASE](https://image.slidesharecdn.com/attinducedhepatitis-190401182334/85/Att-induced-hepatitis-pptx-new-4-320.jpg)