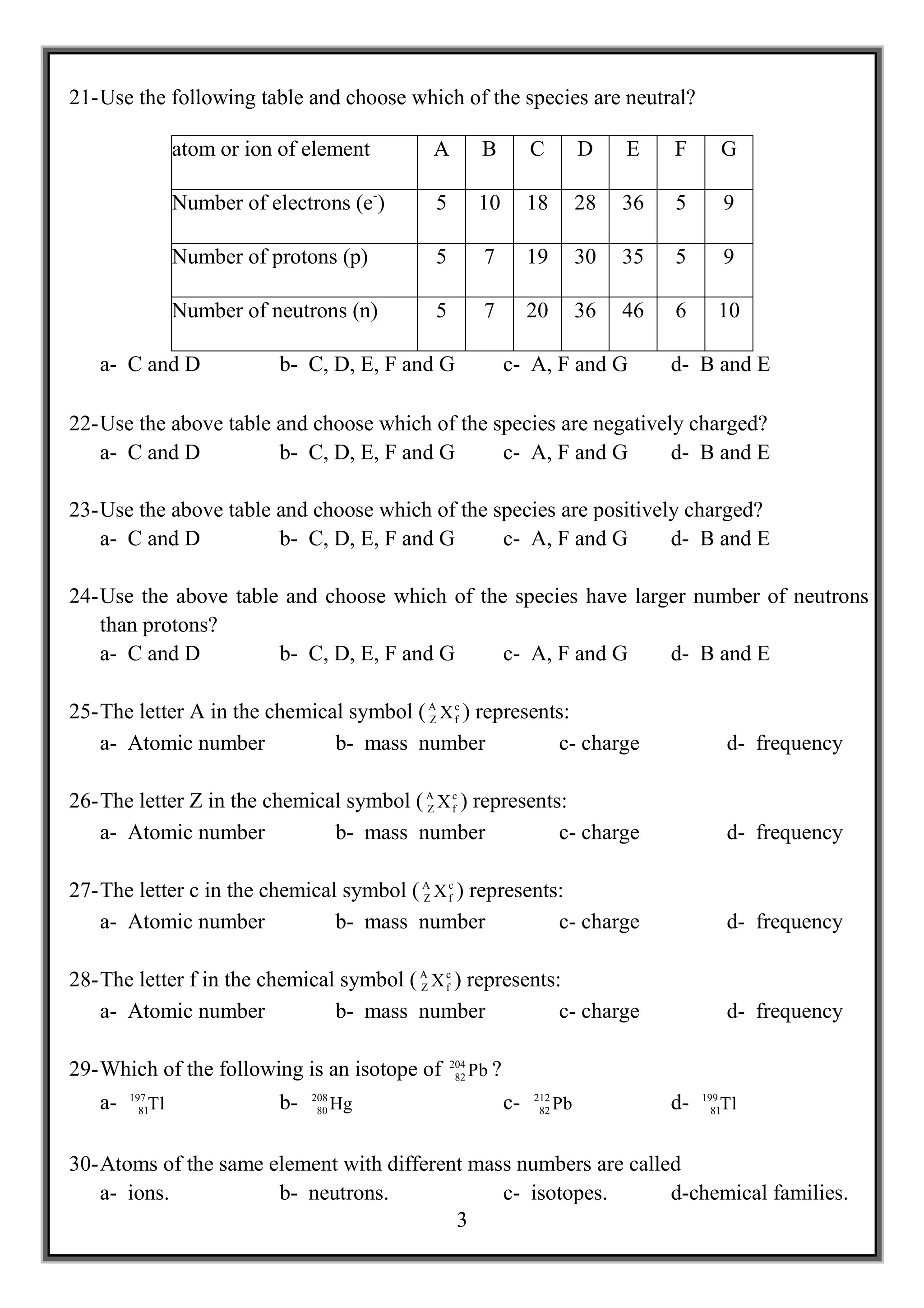
The document contains 69 multiple choice questions about concepts in chemistry including:
1) Scientists who made important discoveries about the structure of atoms such as Thomson, Millikan, Rutherford.
2) Properties of atoms such as mass number, atomic number, isotopes.
3) Ions and their charges.
4) Naming compounds from their formulas and vice versa.
5) Bonding types and common elements and compounds.
The questions assess a wide range of foundational chemistry concepts in a multiple choice format.