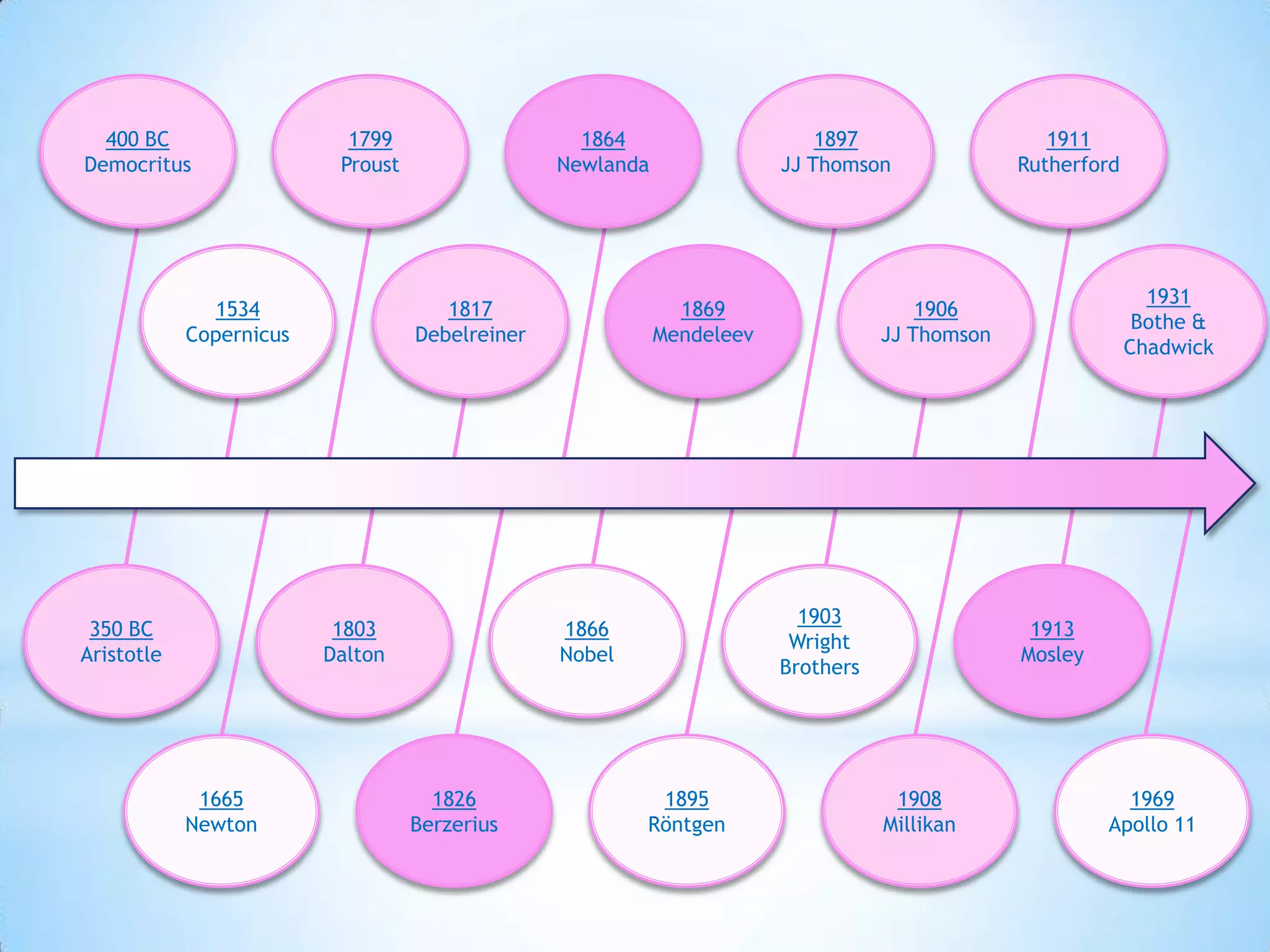
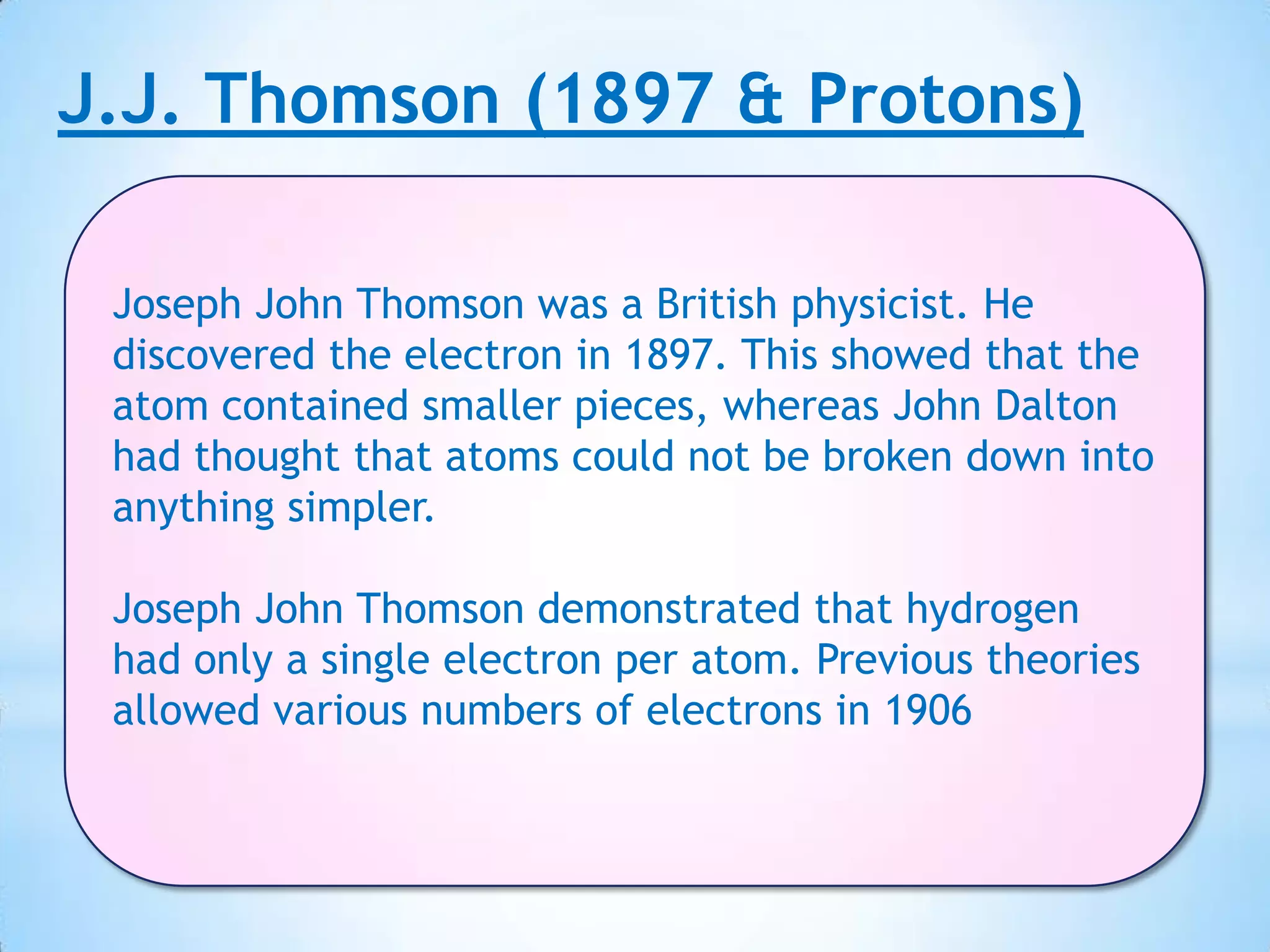
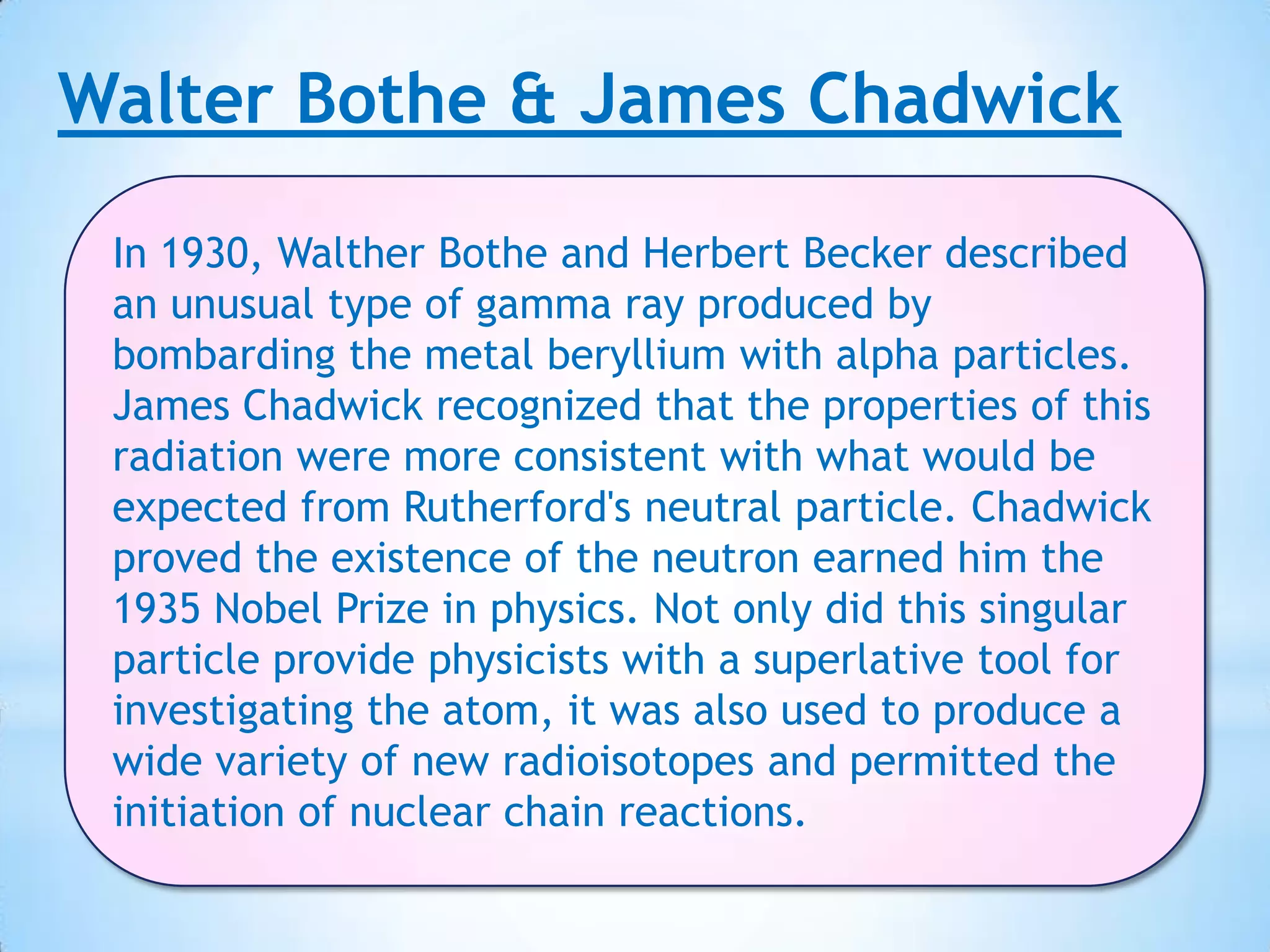
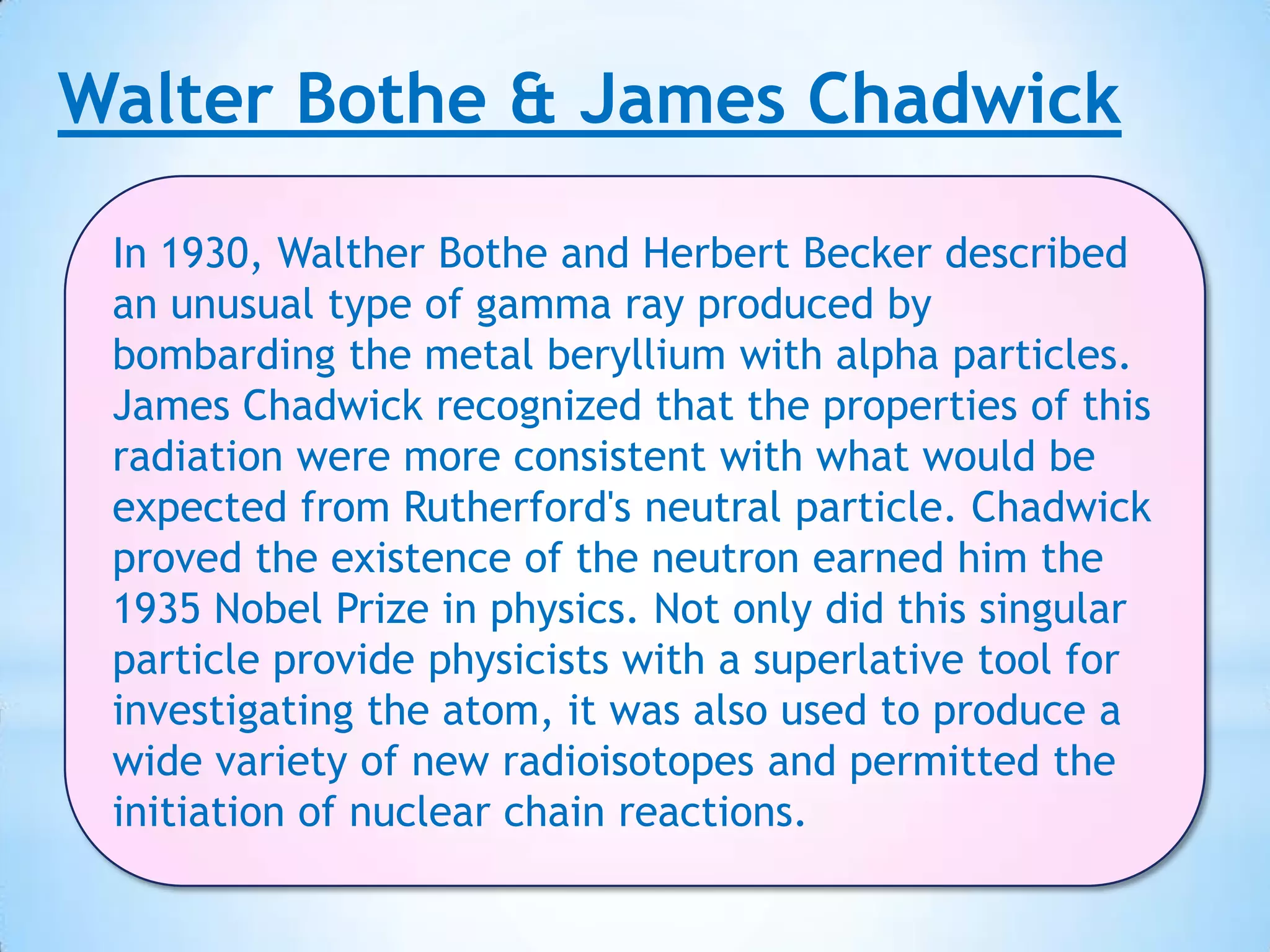
Democritus first proposed that all matter is made up of tiny indivisible particles called atoms in 400 BC, though Aristotle argued matter was infinitely divisible. In the early 19th century, scientists including Proust, Dalton and Döbereiner made discoveries laying the foundations of atomic theory and periodic law. J.J. Thomson discovered the electron in 1897 and proved hydrogen has one electron, while Rutherford proposed the nuclear model of the atom in 1911. Bothe and Chadwick discovered the neutron in 1931, completing modern atomic theory.