Embed presentation
Downloaded 24 times

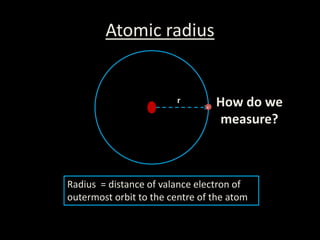
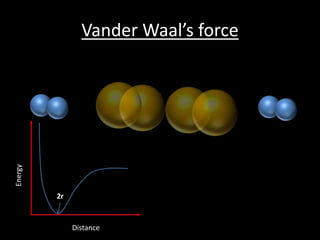
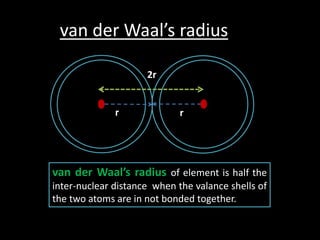
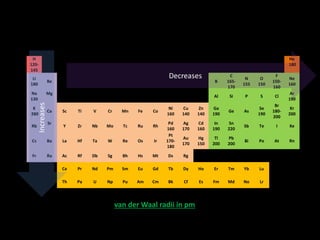
This document discusses atomic radius and van der Waals radius. It defines atomic radius as the distance from the nucleus to the outermost electron and van der Waals radius as half the distance between two unbonded atoms when their electron shells interact. It then provides a periodic table showing van der Waals radii in picometers for each element, with trends of increasing and decreasing radii across periods and down groups.




