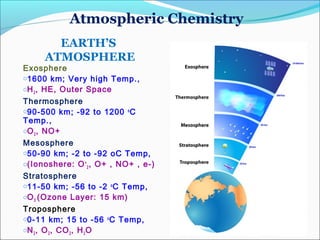
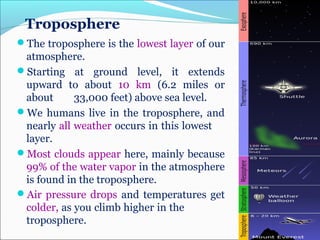
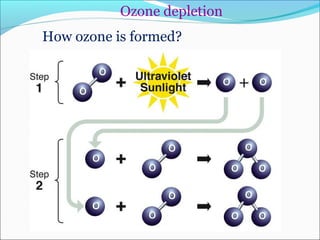
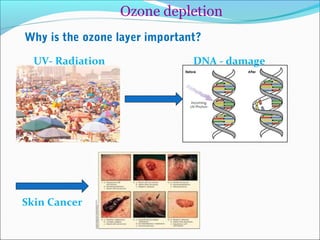
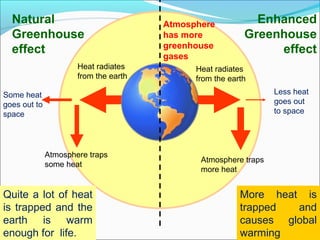
Atmospheric chemistry is the study of the chemistry of Earth's atmosphere and the atmosphere of other planets. It is a multidisciplinary field that draws from various areas including environmental chemistry, physics, meteorology, computer modeling, oceanography, geology, and volcanology. The Earth's atmosphere consists of different layers - the troposphere, stratosphere, mesosphere, thermosphere, and exosphere - each with unique characteristics and compositions. Atmospheric chemistry studies how the composition of the atmosphere changes through natural processes as well as human activities, which can negatively impact human health, ecosystems, and climate through issues like acid rain, ozone depletion, smog, and global warming.