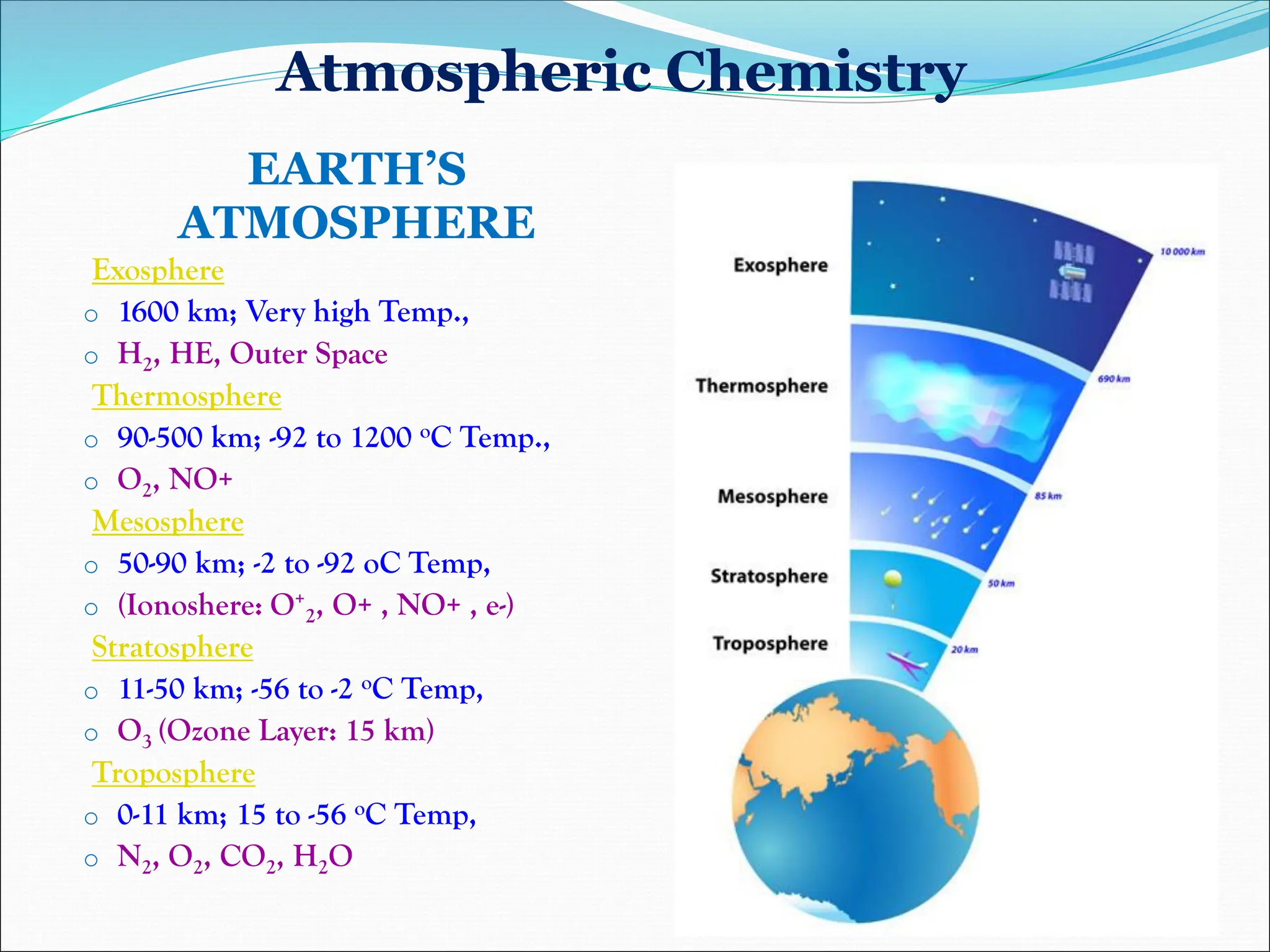
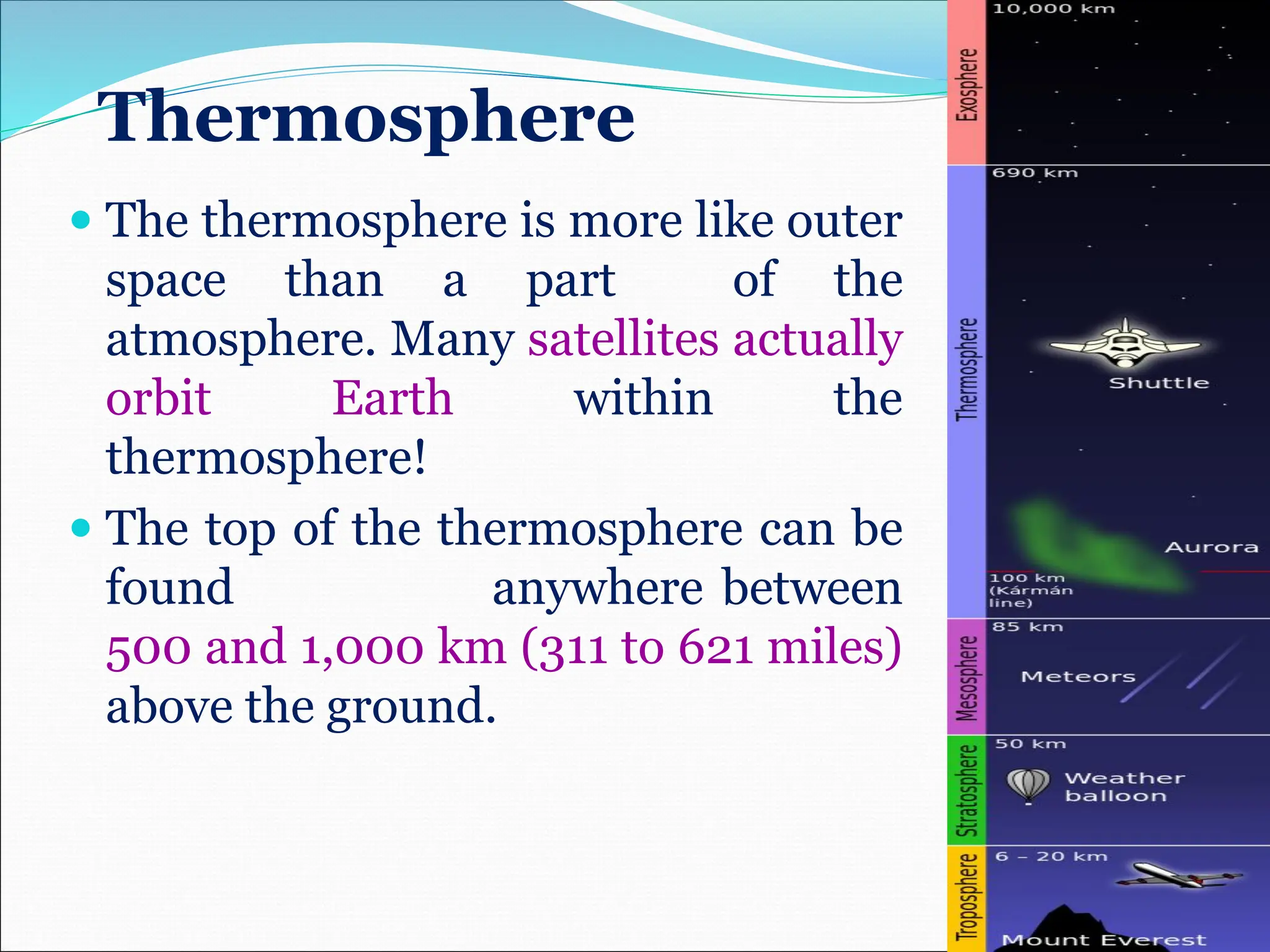
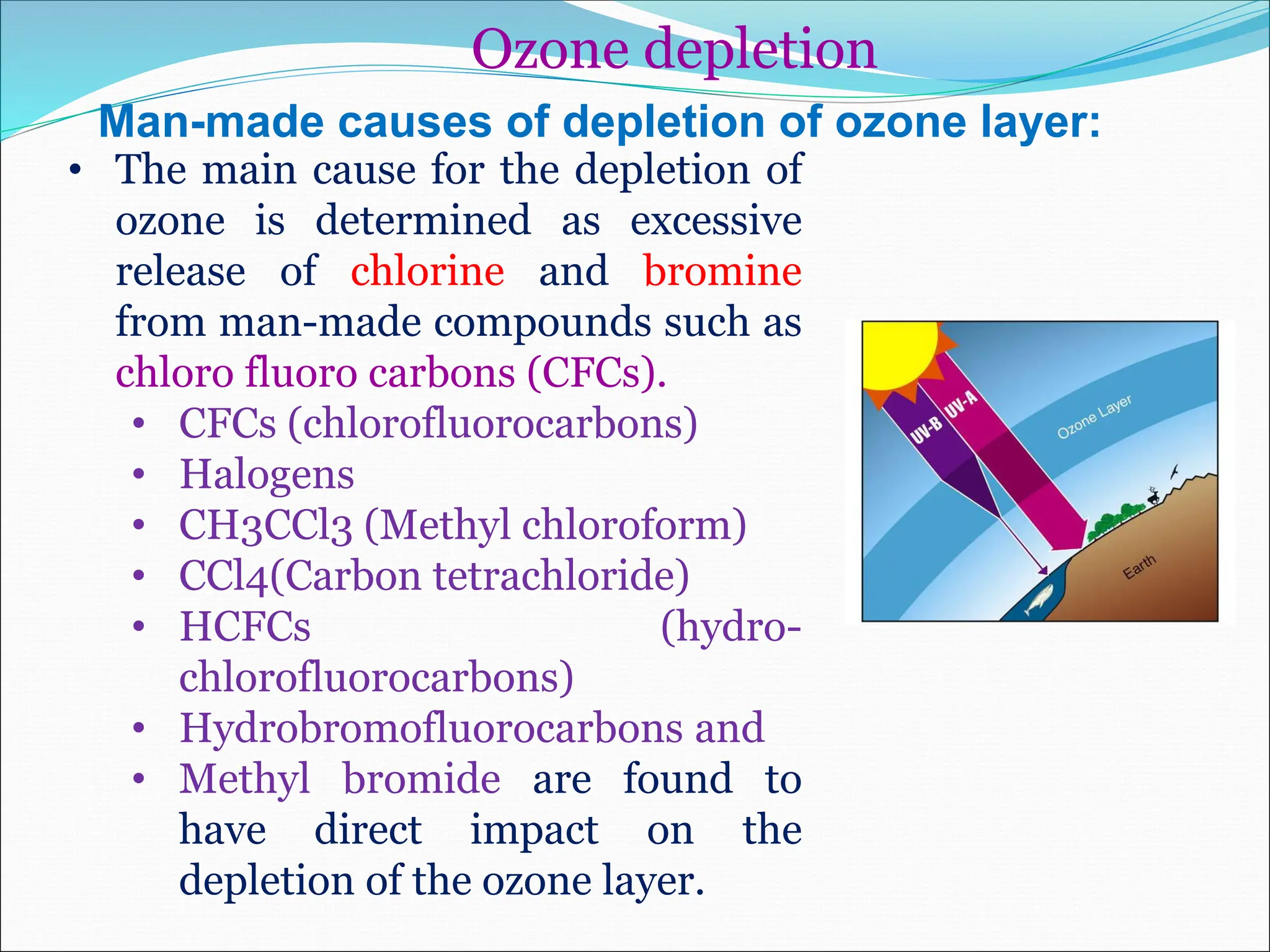
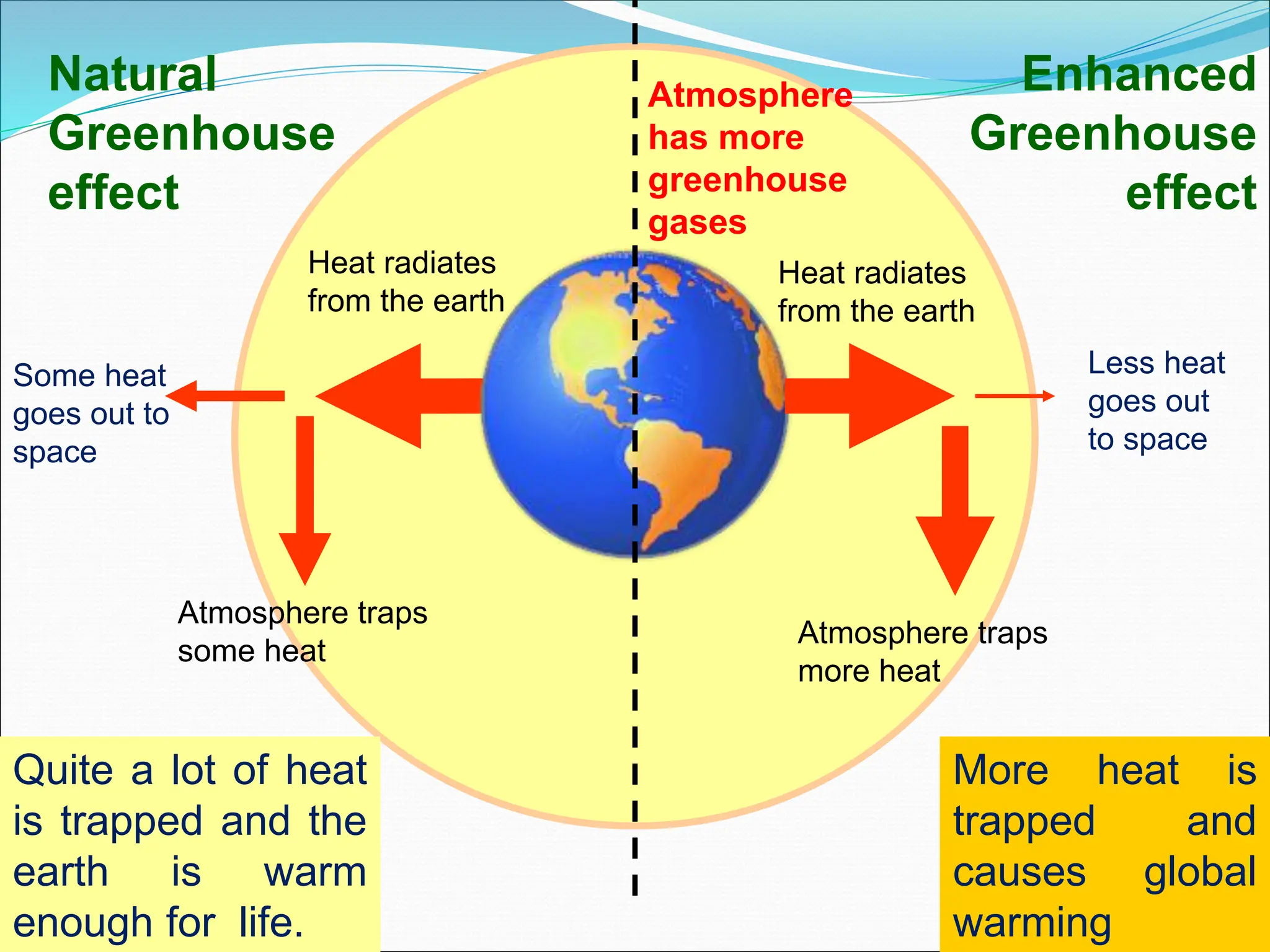
Atmospheric chemistry studies the composition and interactions of gases in the Earth's atmosphere and beyond, with layers categorized as troposphere, stratosphere, mesosphere, thermosphere, and exosphere. Key phenomena include acid rain, ozone depletion from human-made chemicals, and the greenhouse effect, which is exacerbating global warming. Understanding atmospheric chemistry is crucial for addressing environmental issues that impact human health, ecosystems, and climate.