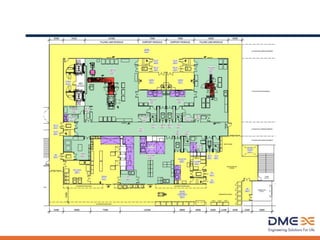
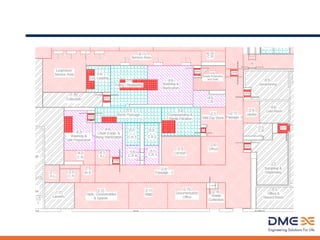
This document provides an overview of sterile liquids manufacturing, including key tenets, requirements, processes, and design considerations. The main points are:
1) Sterile manufacturing requires clean air, water, facilities, equipment, validated processes, and quality control to successfully produce sterile products. Closed processing methods are preferred for product protection.
2) Key unit operations include formulation, filling, stoppering, lyophilization, inspection, and terminal sterilization. Facilities must consider production needs like batch size and container types.
3) Designing sterile facilities requires meeting regulations while addressing safety, containment, utilities, and automation needs to ensure robust and compliant manufacturing.