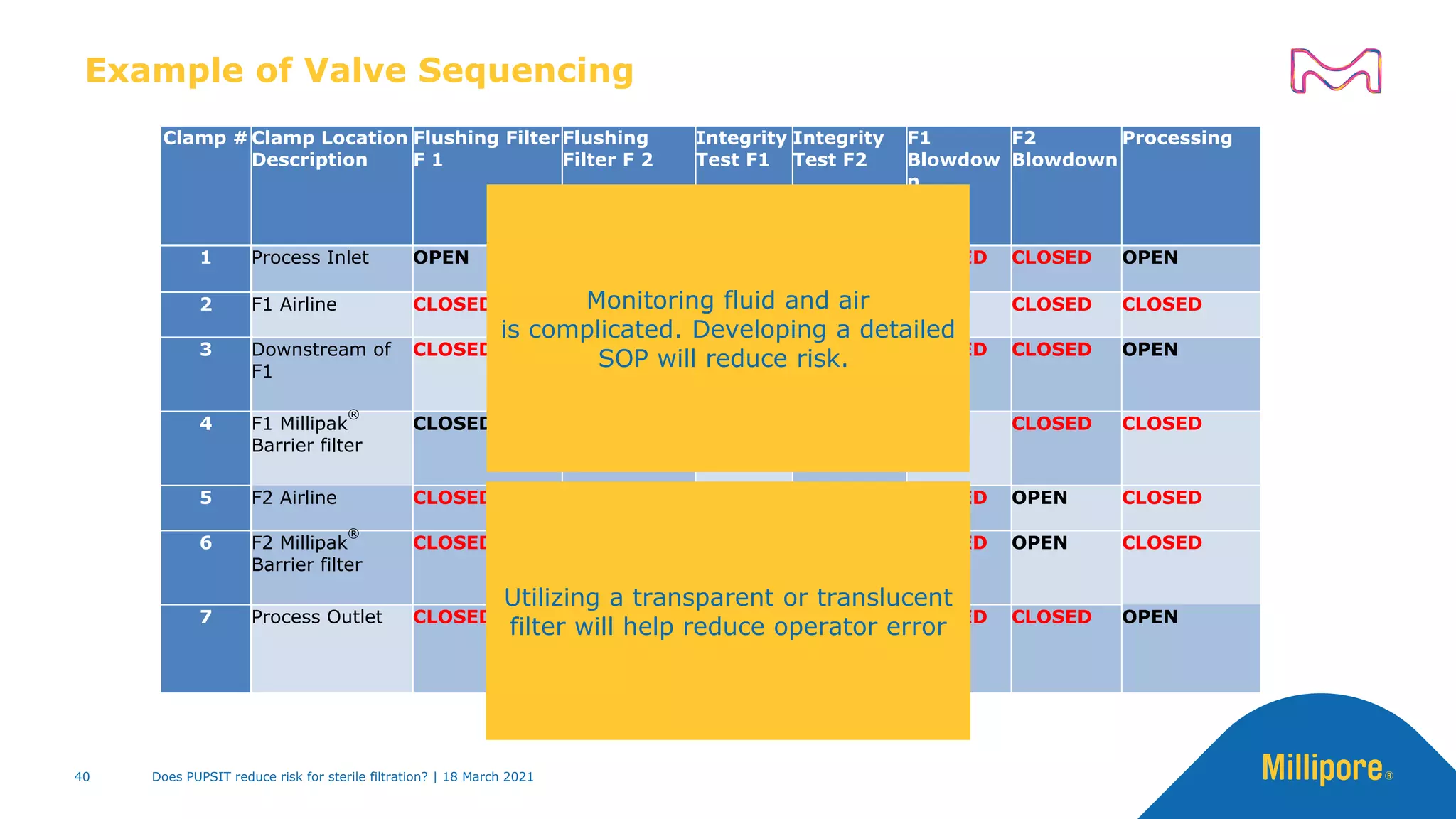
This presentation discusses pre-use post-sterilization integrity testing (PUPSIT) of sterile filters. It provides background on regulatory guidance for integrity testing critical filters before and after use. Masking studies were conducted where flawed filters were exposed to blocking solutions, and in some cases the flaws were able to mask and pass post-use integrity testing. The risk of masking was found to be highly dependent on process conditions like blocking rate and solution concentration. The presentation discusses considerations for final filtration assembly design to enable PUPSIT like using redundant filters and multi-purpose ports. It addresses challenges with maintaining sterility and pressure during PUPSIT.







![Regulatory -
EU GMP Annex 1 draft, 2020:
Integrity test before use and
post-use
“…[PUPSIT] may not always
be possible after sterilization due
to process constraints (e.g. the
filtration of very small volumes of
solution). In these cases, an
alternative approach may be taken
as long as a thorough risk
assessment has been performed
and compliance is achieved by the
implementation of appropriate
controls to navigate any risk of
non-sterility.
Industry -
PDA Technical Report 26:
“Integrity testing alone is
insufficient to assure the sterility
of the filtrate. At least two other
elements must be in place:
The production controls and
quality assurance systems
used by the filter
manufacturer ...
And the validation studies
used to show that a particular
combination of product,
processing conditions and
sterilizing grade filter will meet
the requirements of the
bacterial challenge test.”
Establish minimum
Bubble Point
Membrane manufacturing
and testing
Device manufacturing
and testing
Validation studies
End-user integrity test
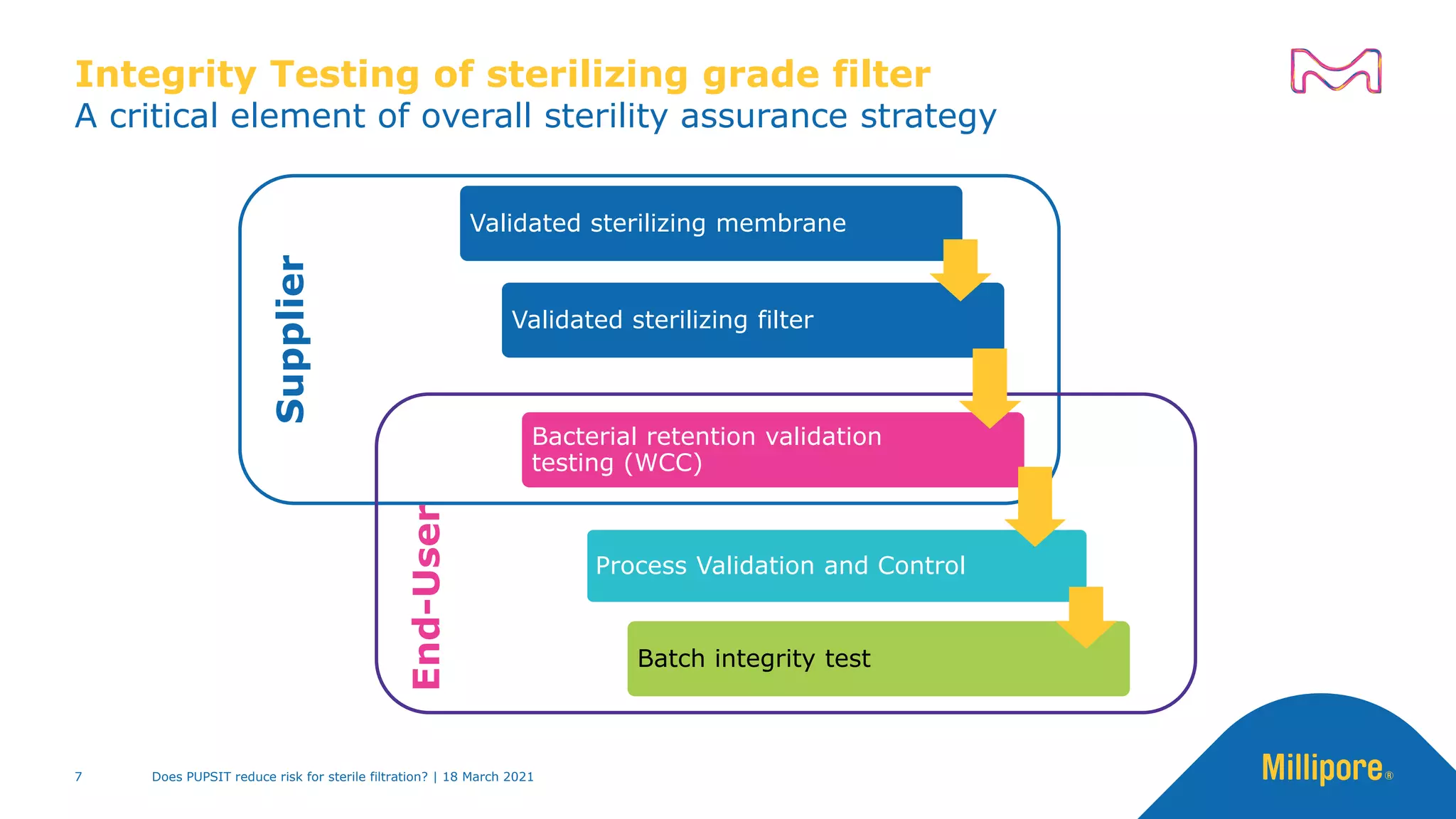
Integrity test of "sterilizing filter” alone is insufficient
Evolving Perspective – Sterility Assurance
Does PUPSIT reduce risk for sterile filtration? | 18 March 2021
12](https://image.slidesharecdn.com/pupsitwebinarfinalmarch18-210319092119/75/Does-PUPSIT-Reduce-Risk-for-Sterile-Filtration-12-2048.jpg)