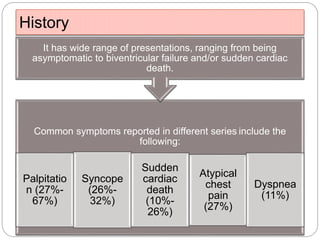
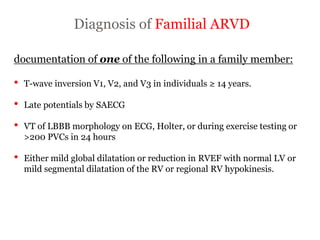
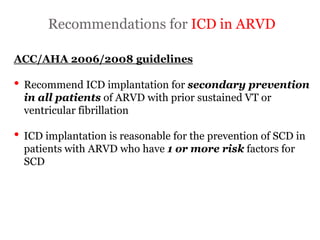
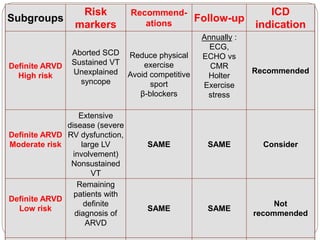
1) ARVD is a genetic cardiomyopathy characterized by replacement of RV myocardium by fat and fibrosis. It is diagnosed using criteria in 5 categories: RV structure/function by echo/CMR, RV biopsy, ECG repolarization/depolarization abnormalities, arrhythmias, and family history.
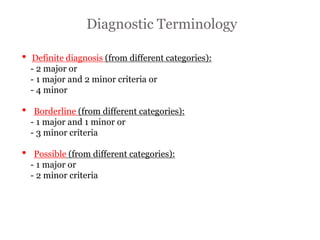
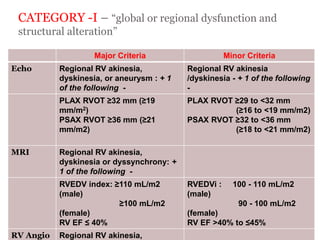
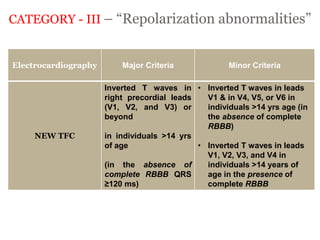
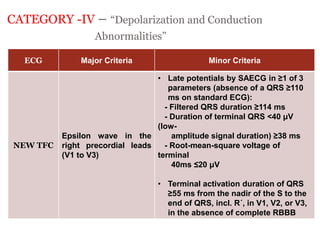
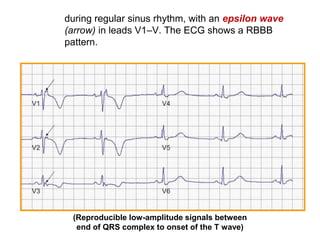
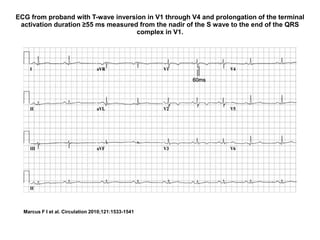
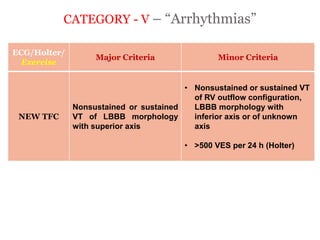
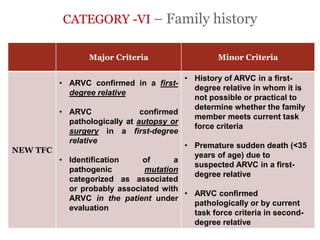
2) Major diagnostic criteria include RV akinesia/aneurysm by echo/CMR, >500 ventricular extrasystoles per 24 hours by Holter, nonsustained or sustained VT of left bundle branch block morphology, and epsilon waves on ECG. Meeting criteria in different categories is needed for a definite diagnosis.
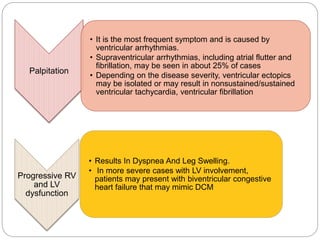
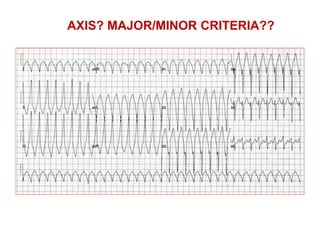
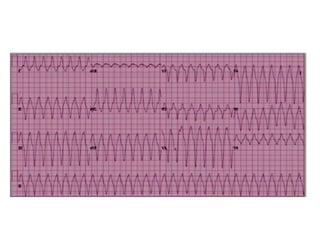
3) Exercise commonly provokes ventricular arrhythmias in ARVD patients, likely due










![Combined endocardial and epicardial
substrate guided catheter ablation
Epicardial scar is wider than the endocardial scar
in ARVD
Combined endocardial & epicardial substrate
guided ablation resulted in a very good short-
and mid-term success rate.
The high recurrence rate published in earlier
series may be due to the conventional only-[Combined endocardial and epicardial catheter ablation in arvc. Brugada J.; Circulation: Arrhythmia and EP.
2012;5:111-121]](https://image.slidesharecdn.com/arvd-drprithvipuwar-160614064625/85/Arvd-dr-prithvi-puwar-46-320.jpg)
