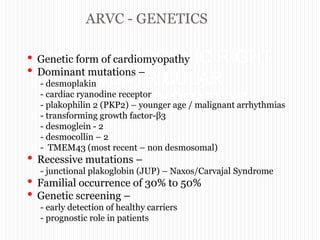
This document discusses arrythmogenic right ventricular cardiomyopathy (ARVC). It begins by explaining the genetics of ARVC, noting that mutations can be either dominant or recessive. It then describes the natural history, clinical presentation, diagnosis, and criteria used to diagnose ARVC based on the revised Task Force Criteria. This includes major and minor criteria in categories such as imaging, electrocardiography findings, biopsy results, and family history. The document concludes by discussing management strategies for ARVC including ICD therapy, antiarrhythmic drugs, ablation, heart failure treatment, and transplantation.









































![Combined endocardial and epicardial
substrate guided catheter ablation
Epicardial scar is wider than the endocardial scar
in ARVD
Combined endocardial & epicardial substrate
guided ablation resulted in a very good short- and
mid-term success rate.
The high recurrence rate published in earlier
series may be due to the conventional only-
endocardial approach
[Combined endocardial and epicardial catheter ablation in arvc. Brugada J.;
Circulation: Arrhythmia and EP. 2012;5:111-121]](https://image.slidesharecdn.com/arrythmogenicrightventricularcardiomyopathyppt-140725045755-phpapp02/85/ARVD-Arrythmogenic-right-ventricular-cardiomyopathy-updated-task-force-criteria-ppt-45-320.jpg)

