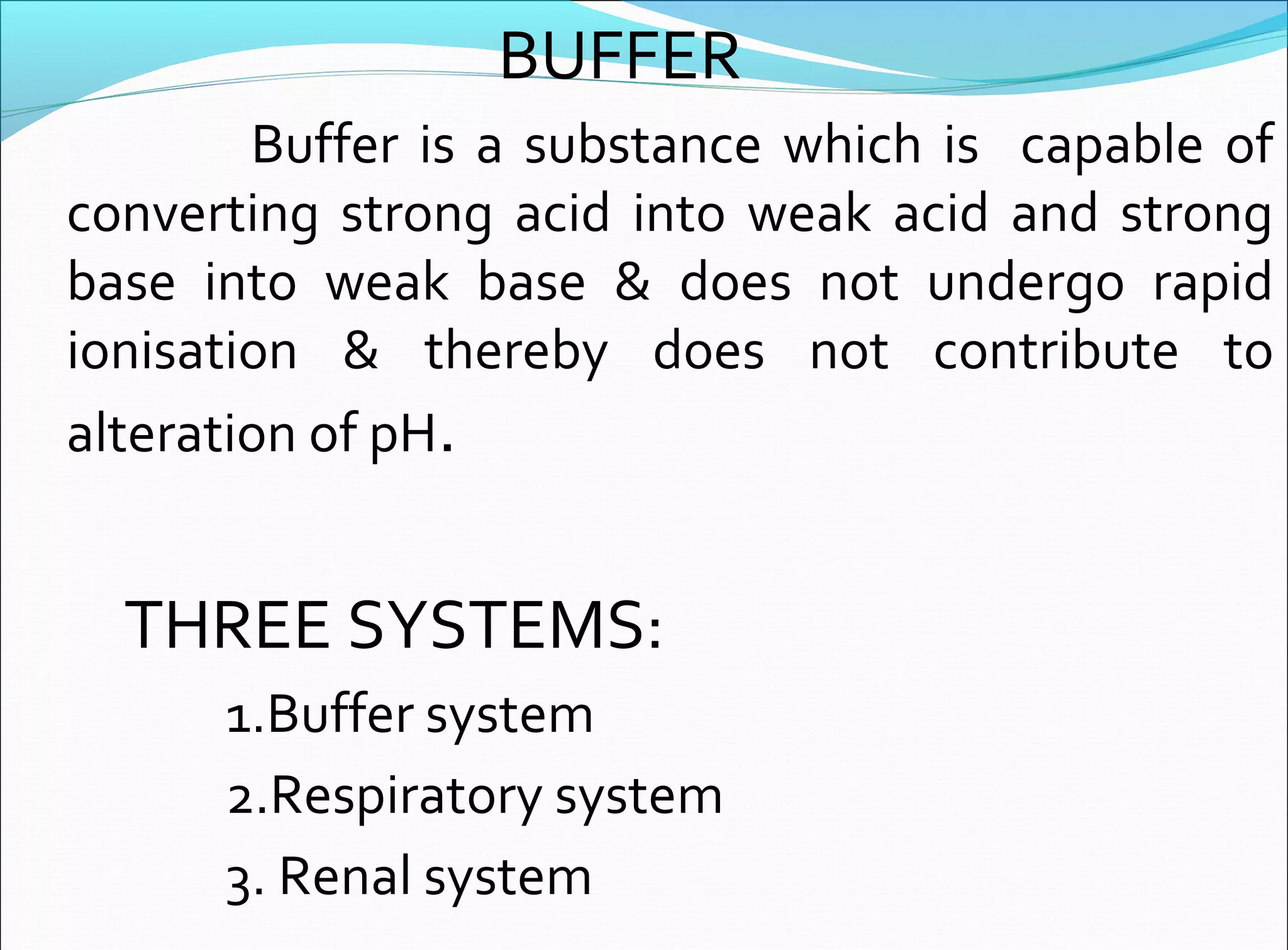
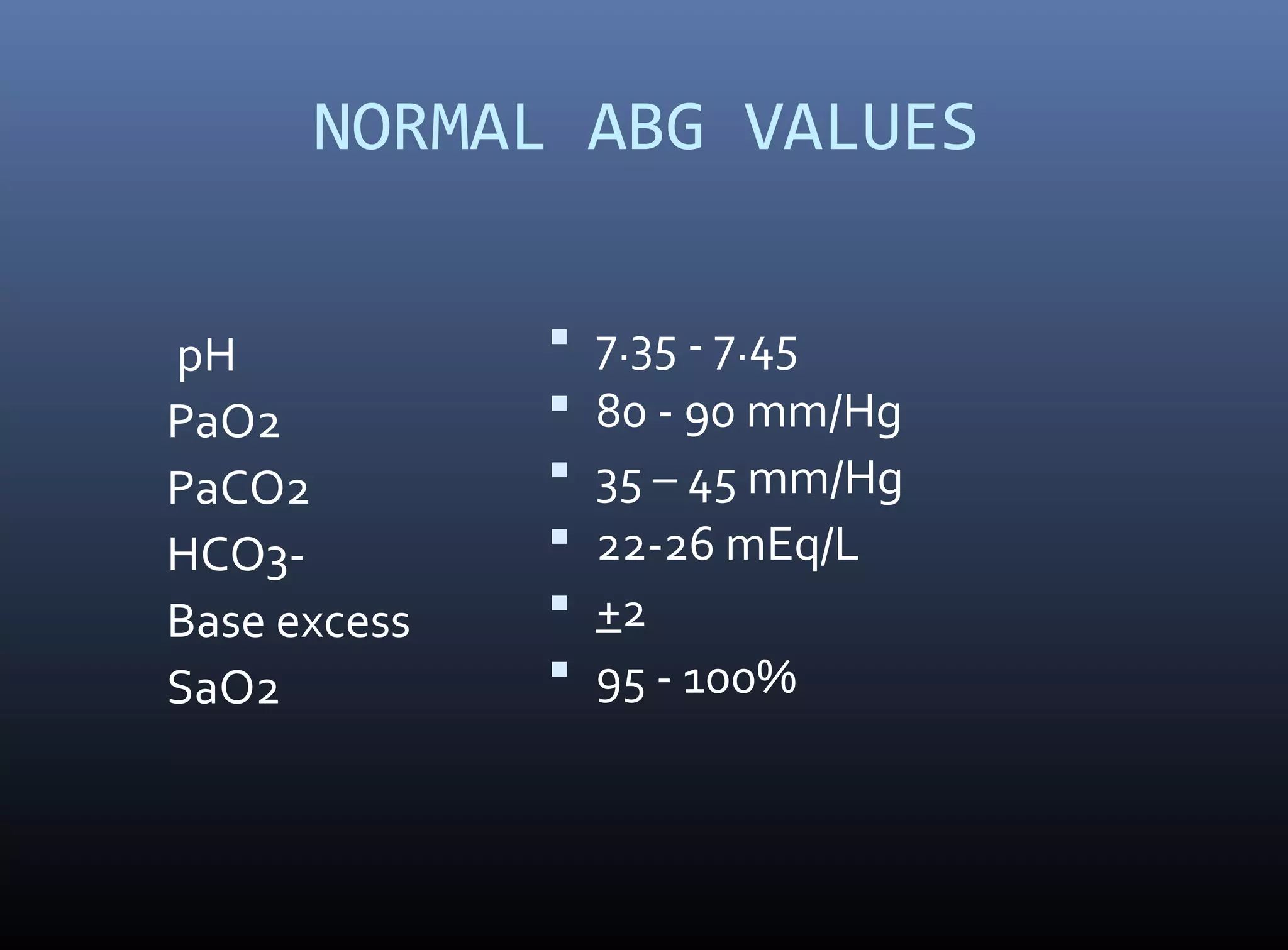
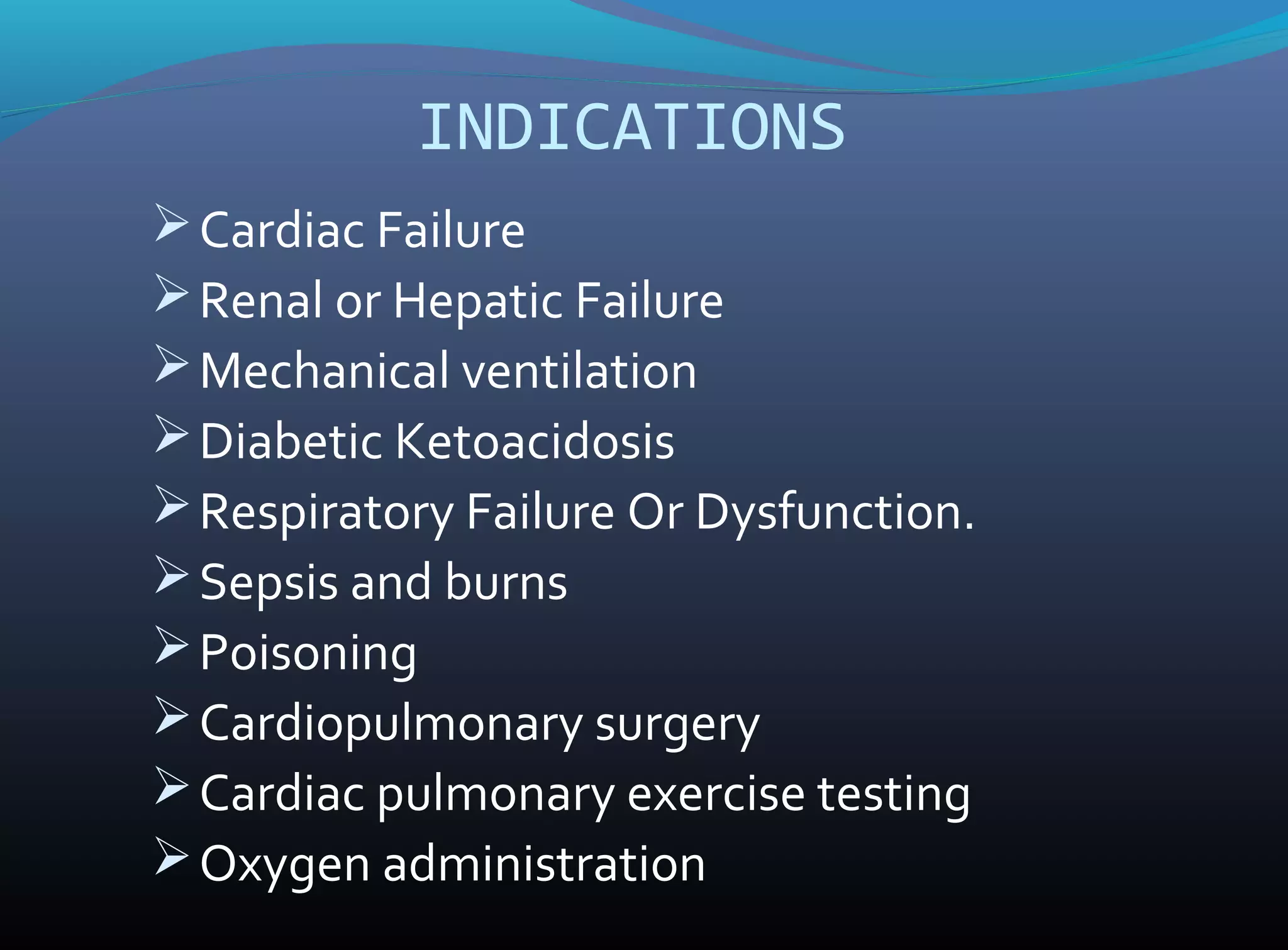
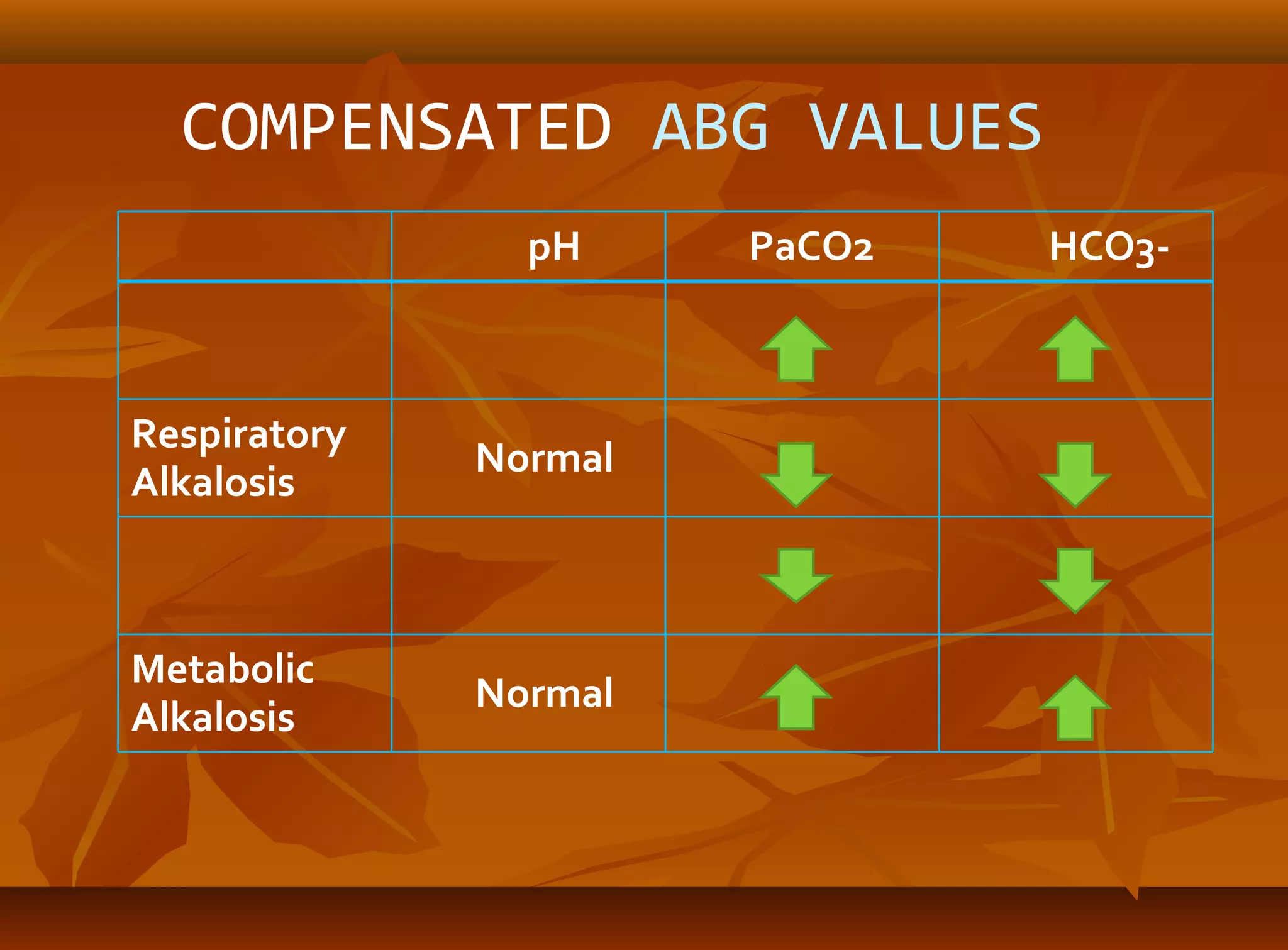
An arterial blood gas (ABG) analysis measures pH, oxygen, and carbon dioxide levels in the blood to help diagnose respiratory and metabolic conditions. It requires collecting a blood sample directly from an artery, usually the radial artery in the wrist. Normal ABG values include a pH of 7.35-7.45, PaO2 of 80-90 mmHg, PaCO2 of 35-45 mmHg, and HCO3- of 22-26 mEq/L. Complications can include bleeding, hematoma, and infection if not performed correctly. The results are used to identify respiratory issues like hypoxia or acidosis and metabolic derangements.
![ARTERIAL BLOOD GAS ANALYSIS
[ABG]](https://image.slidesharecdn.com/arterialbloodgasinterpretation-140715053456-phpapp01/75/Arterial-blood-gas-interpretation-1-2048.jpg)