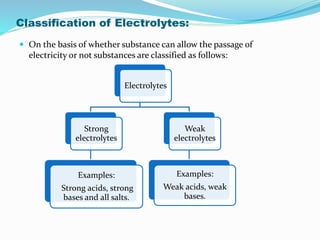
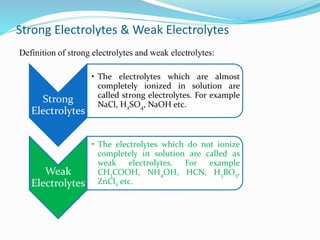
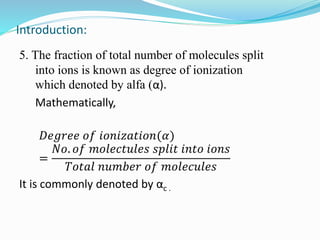
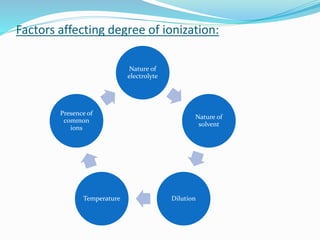
Unit 3 covers the topic of ionic equilibrium, including:
1. The ionization of weak electrolytes and how it follows Ostwald dilution law.
2. The concepts of degree of ionization, ionization constant, and how they relate to the strength of acids and bases in terms of Ka, Kb, pKa and pKb values.
3. Different acid-base concepts including Arrhenius, Bronsted-Lowry, and Lewis concepts.
4. Additional topics like the ionization of water, pH and pH scale, hydrolysis of salts, solubility product principle and its applications, common ion effect, buffer solutions, and solving related numerical problems.