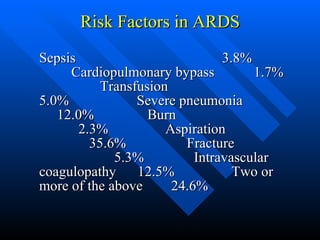
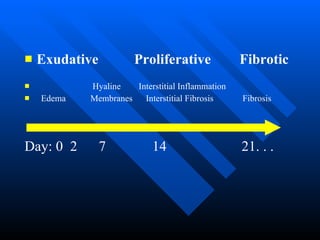
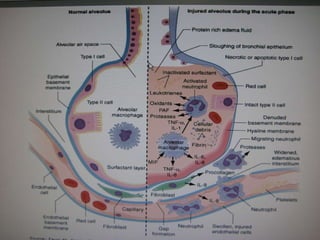
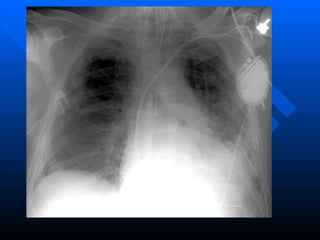
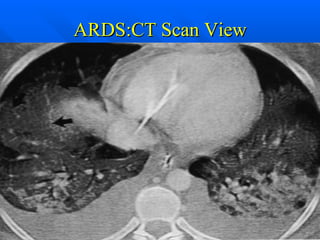
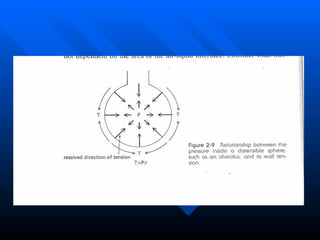
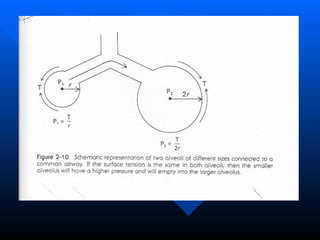
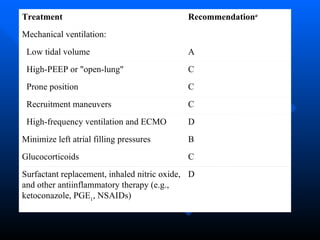
The document summarizes acute respiratory distress syndrome (ARDS), including its definition, risk factors, pathophysiology, clinical presentation, management, and treatment. ARDS is characterized by hypoxemia, bilateral lung infiltrates, and respiratory failure caused by various lung injuries. It involves exudative, proliferative and fibrotic phases. Management includes mechanical ventilation with low tidal volumes, positive end-expiratory pressure, fluid restriction and treatment of underlying conditions. However, mortality remains high at 50-60%.