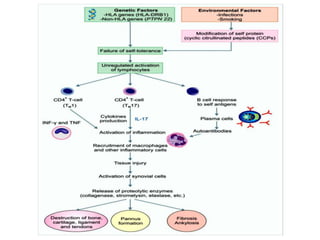
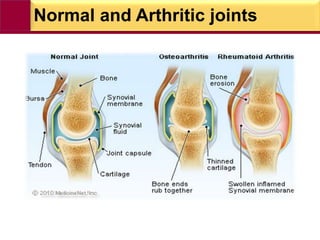
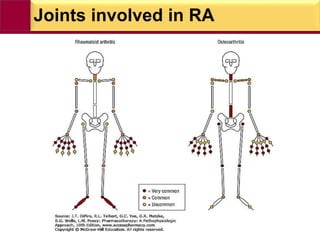
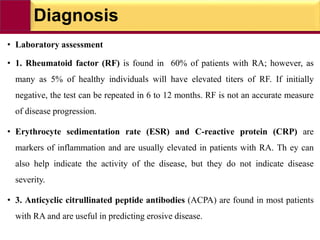
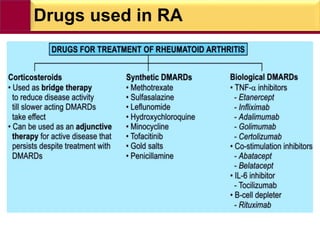
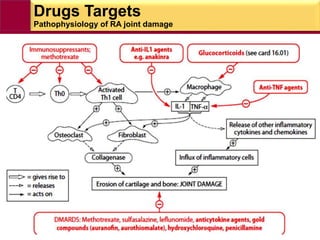
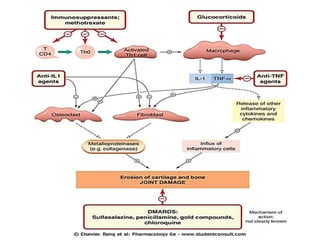
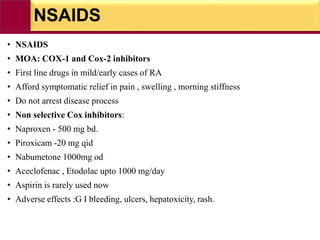
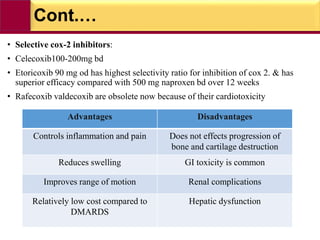
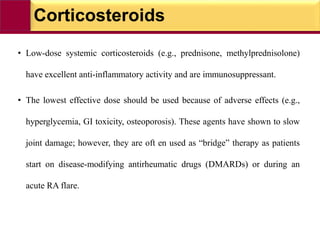
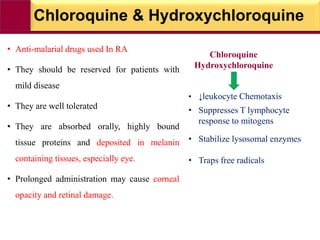
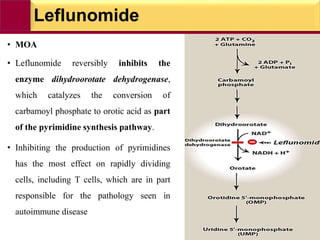
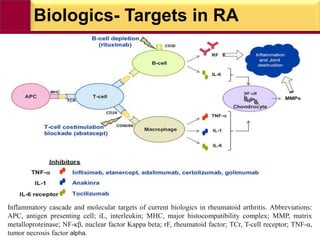
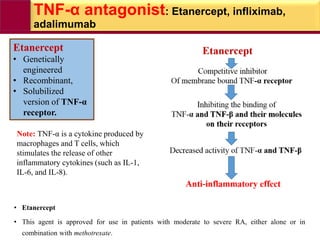
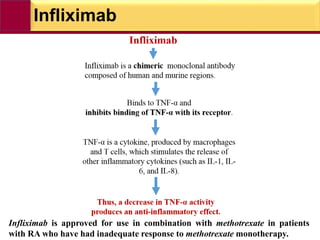
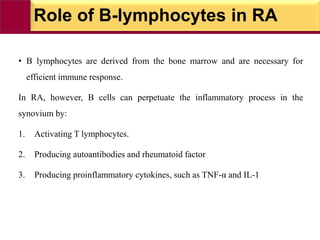
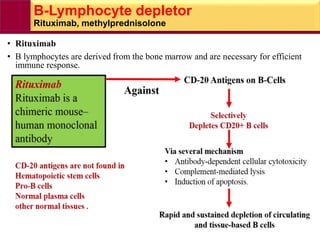
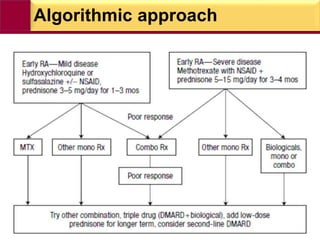
This document provides information on rheumatoid arthritis (RA) including its symptoms, causes, pathophysiology, diagnosis, and treatment. It discusses how RA is a chronic autoimmune disease that causes inflammation of the joints and can affect internal organs. Treatment involves NSAIDs, corticosteroids, and disease-modifying antirheumatic drugs (DMARDs) like methotrexate, sulfasalazine, hydroxychloroquine, and biologic drugs that target cytokines like TNF-alpha. The goals of treatment are to reduce joint damage, pain, disability and maintain quality of life while minimizing adverse effects.