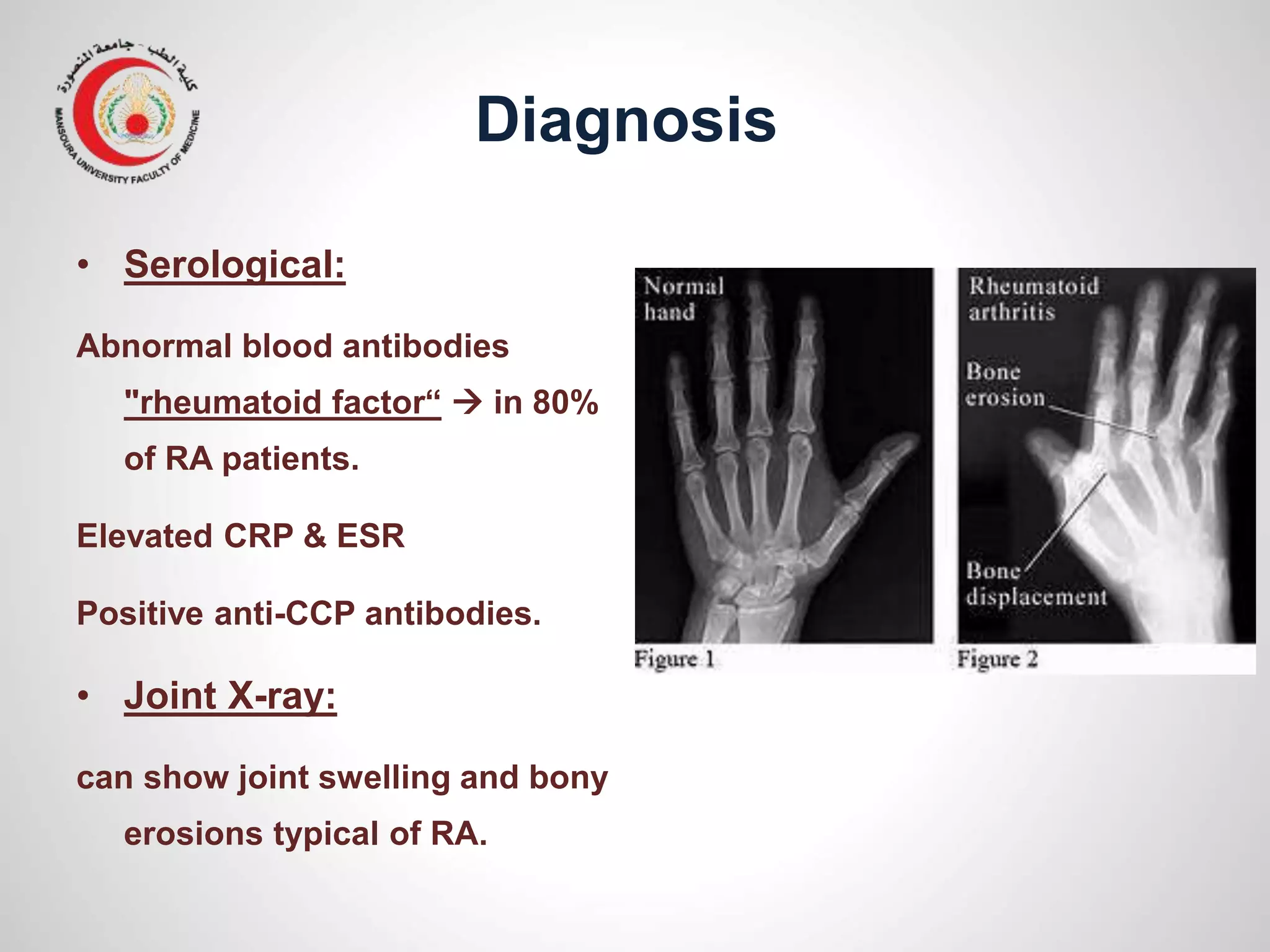
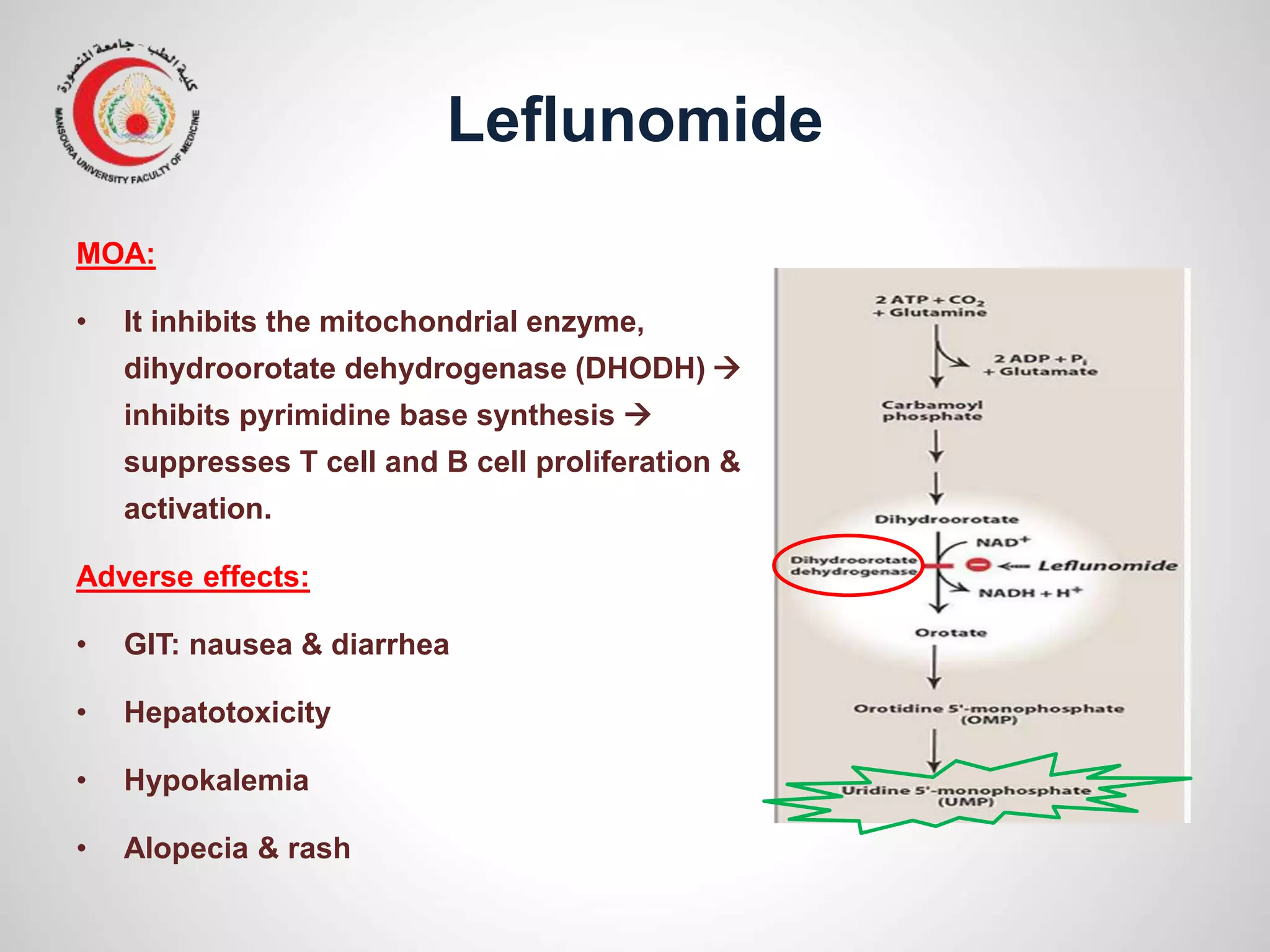
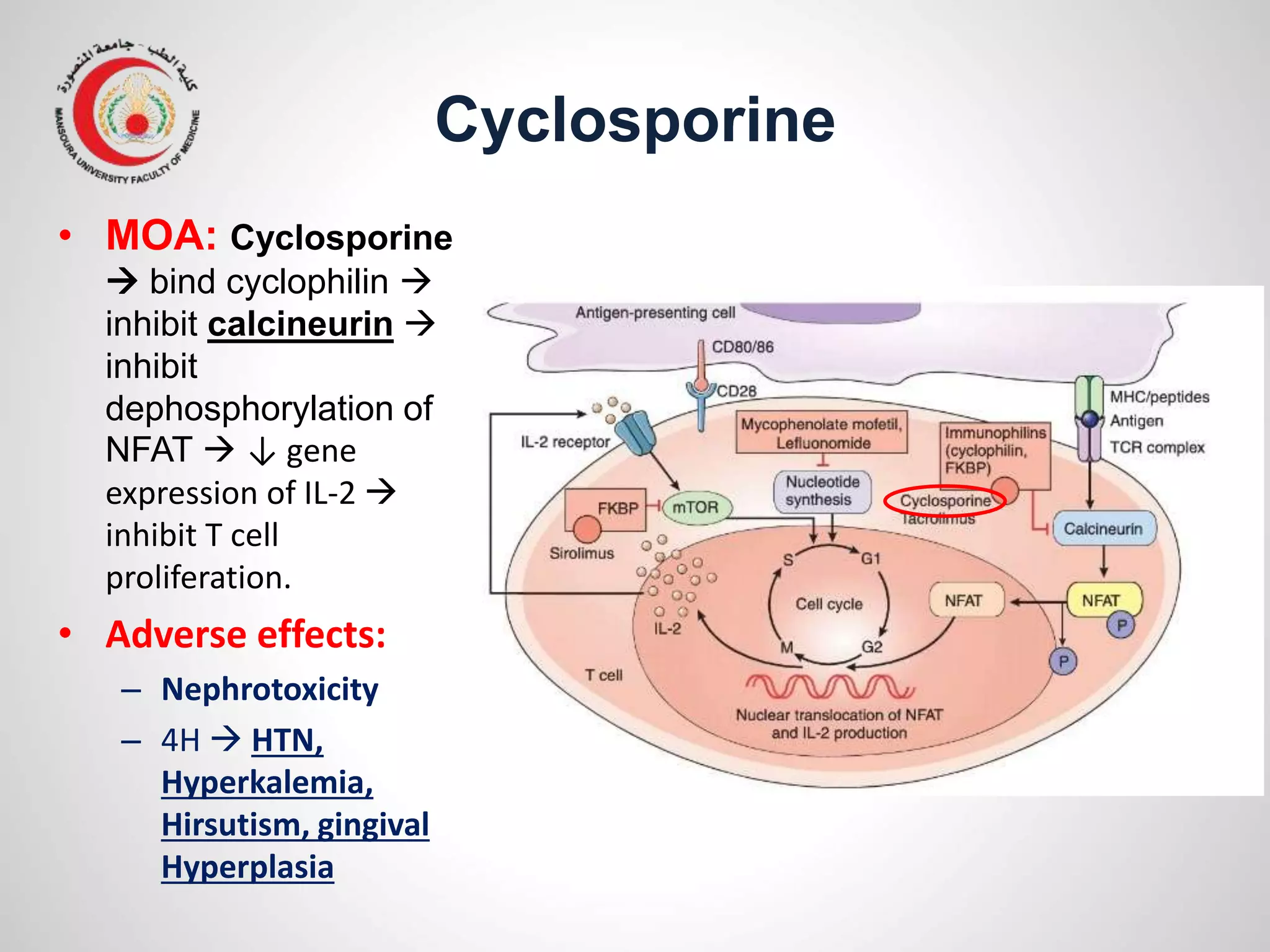
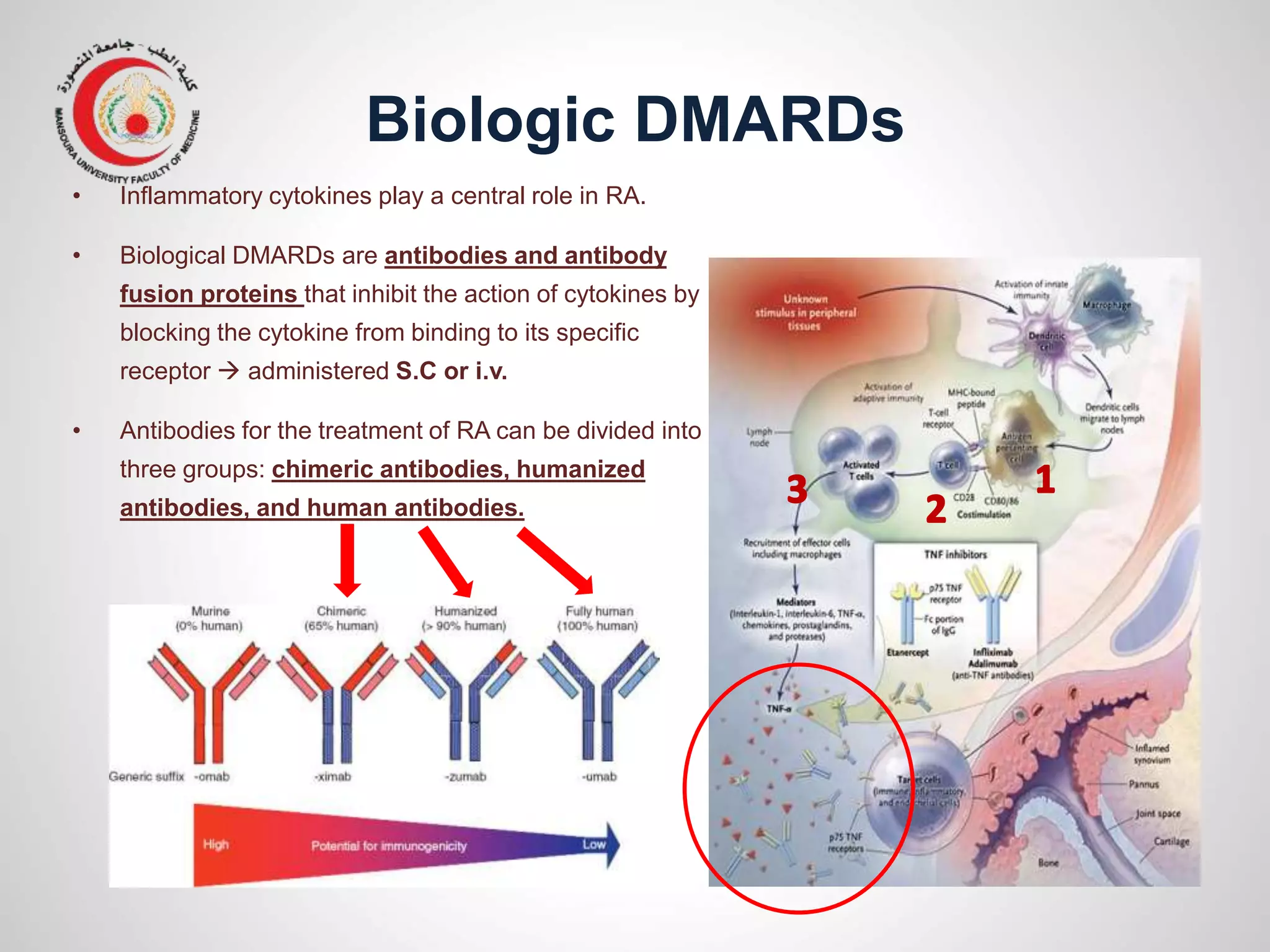
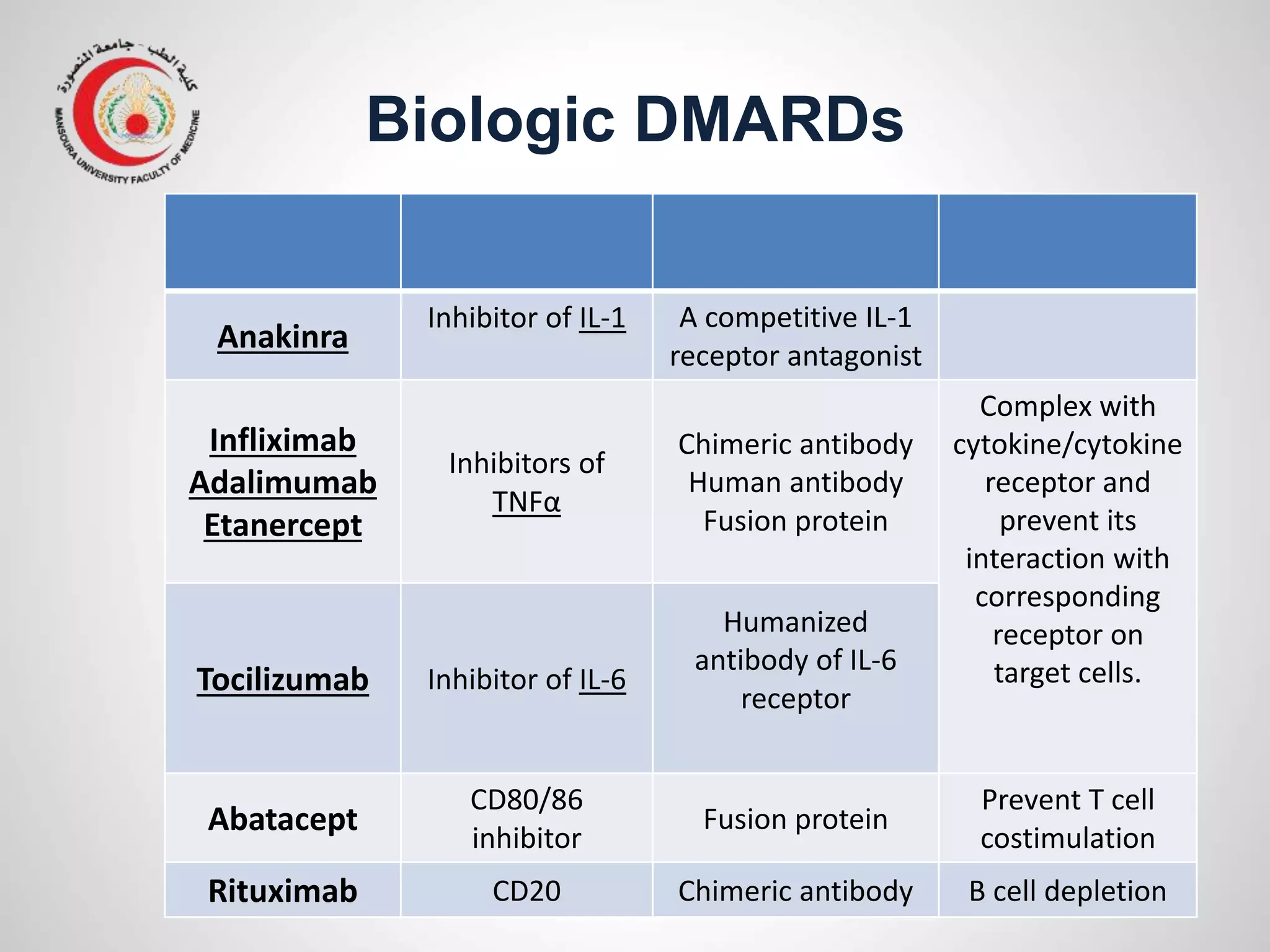
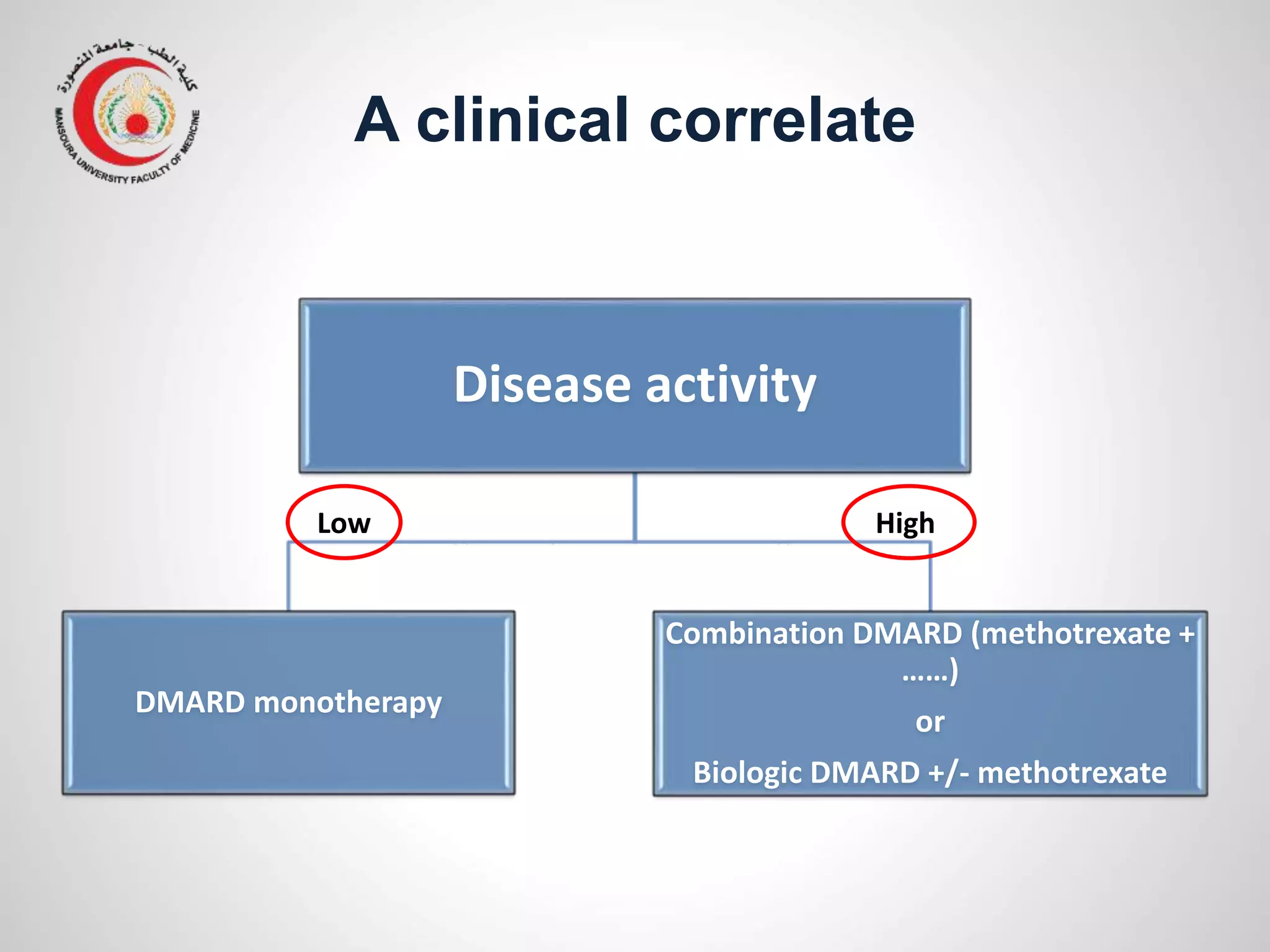
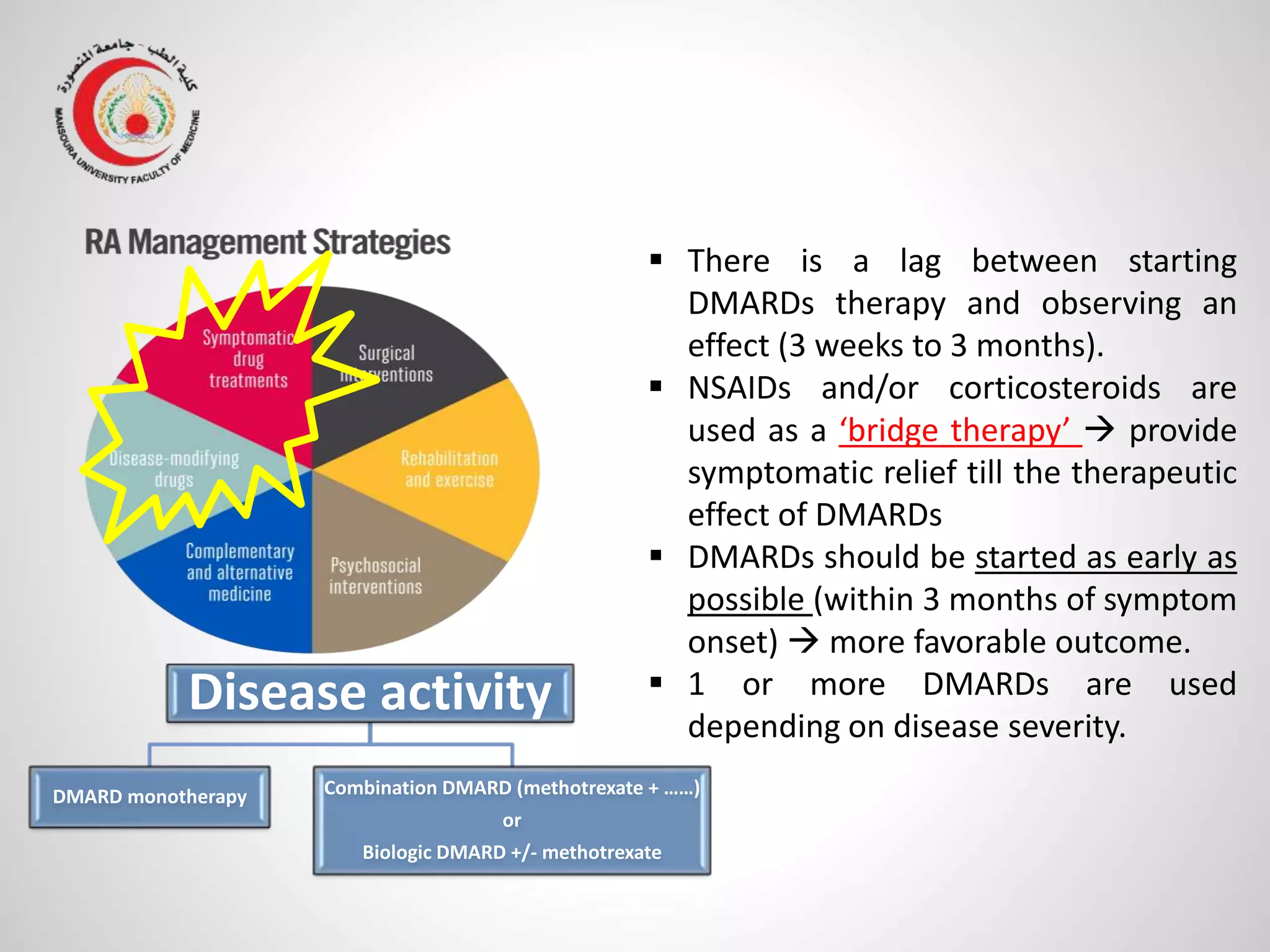
This document provides information on the treatment of rheumatoid arthritis (RA) including disease-modifying anti-rheumatic drugs (DMARDs). It discusses the case of a 58-year-old lady with a 3-year history of RA who is experiencing a flare up. Her treatment options include starting DMARD monotherapy or combination therapy depending on her disease activity level to prevent further joint destruction while also using steroids for symptomatic relief. DMARDs like methotrexate work by modifying the immune system to slow disease progression and should be started early for best outcomes.