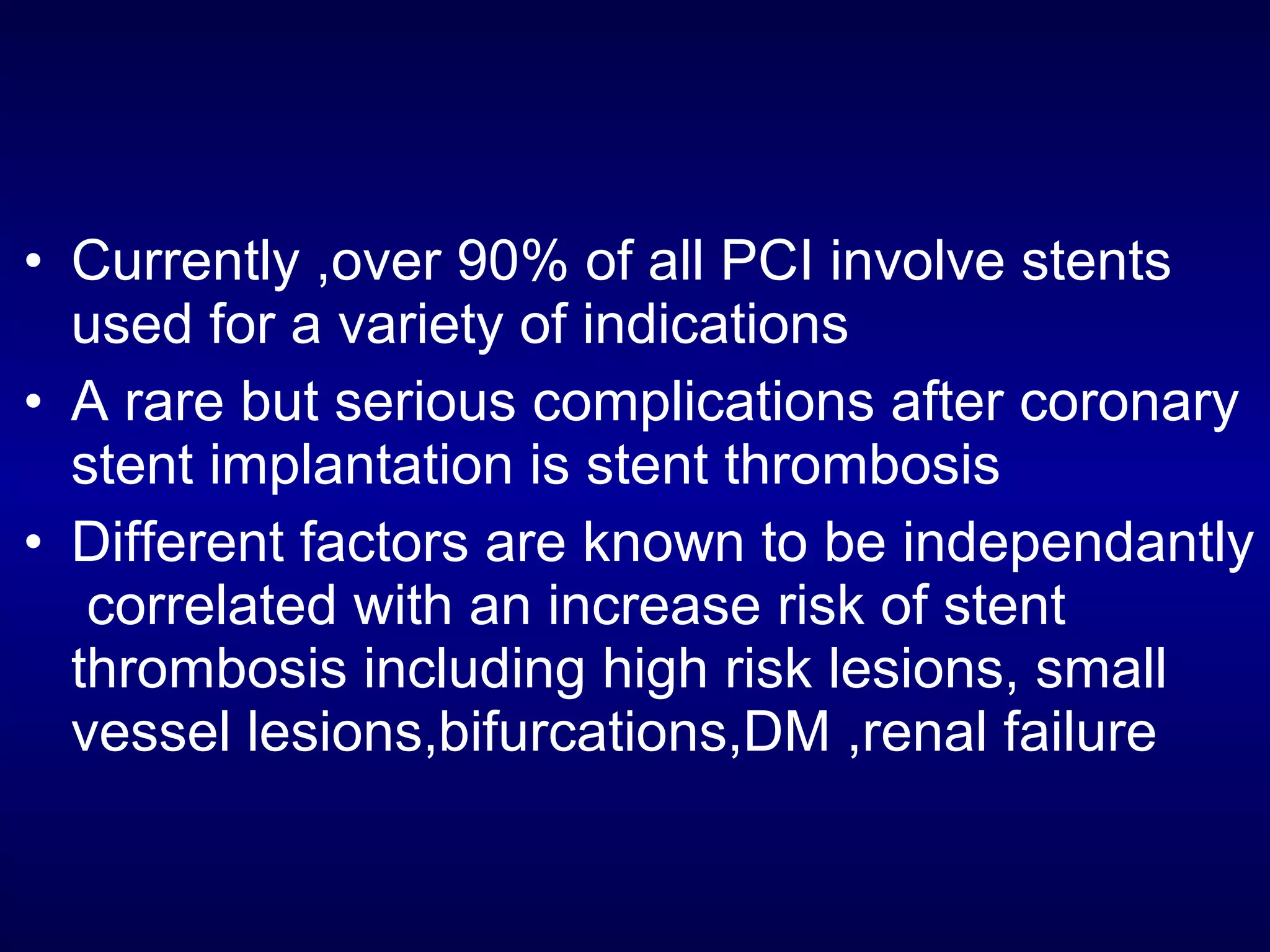
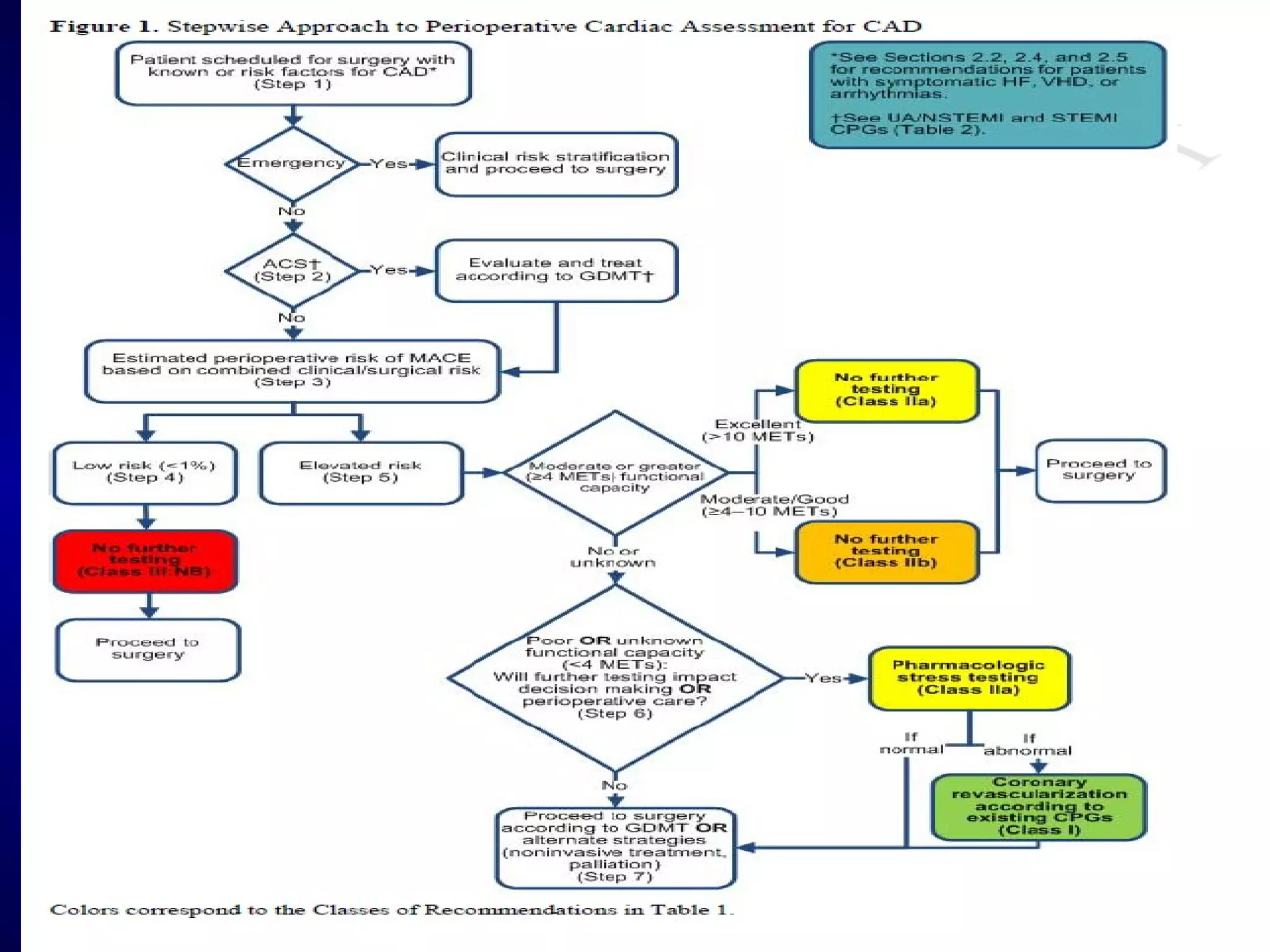
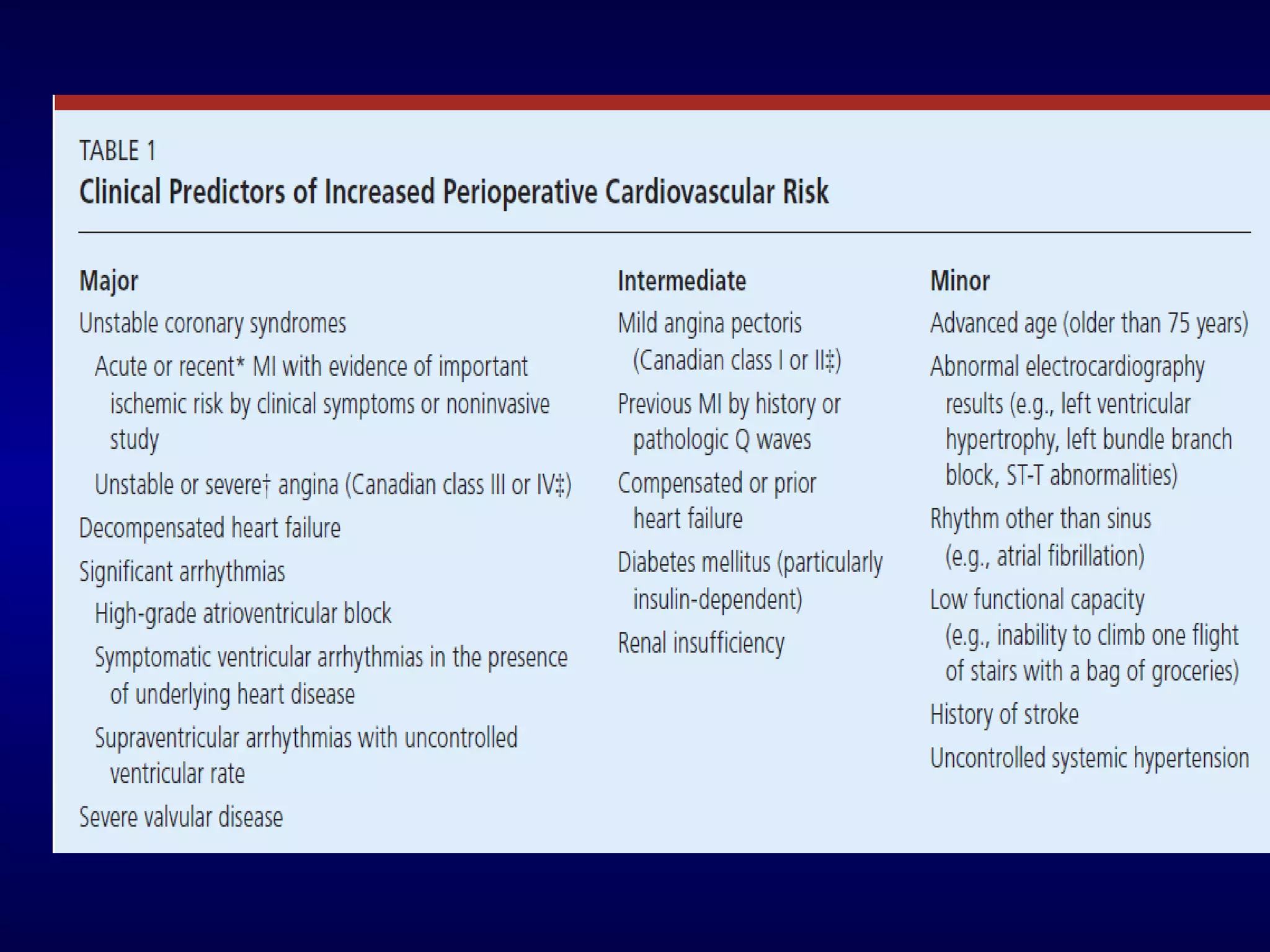
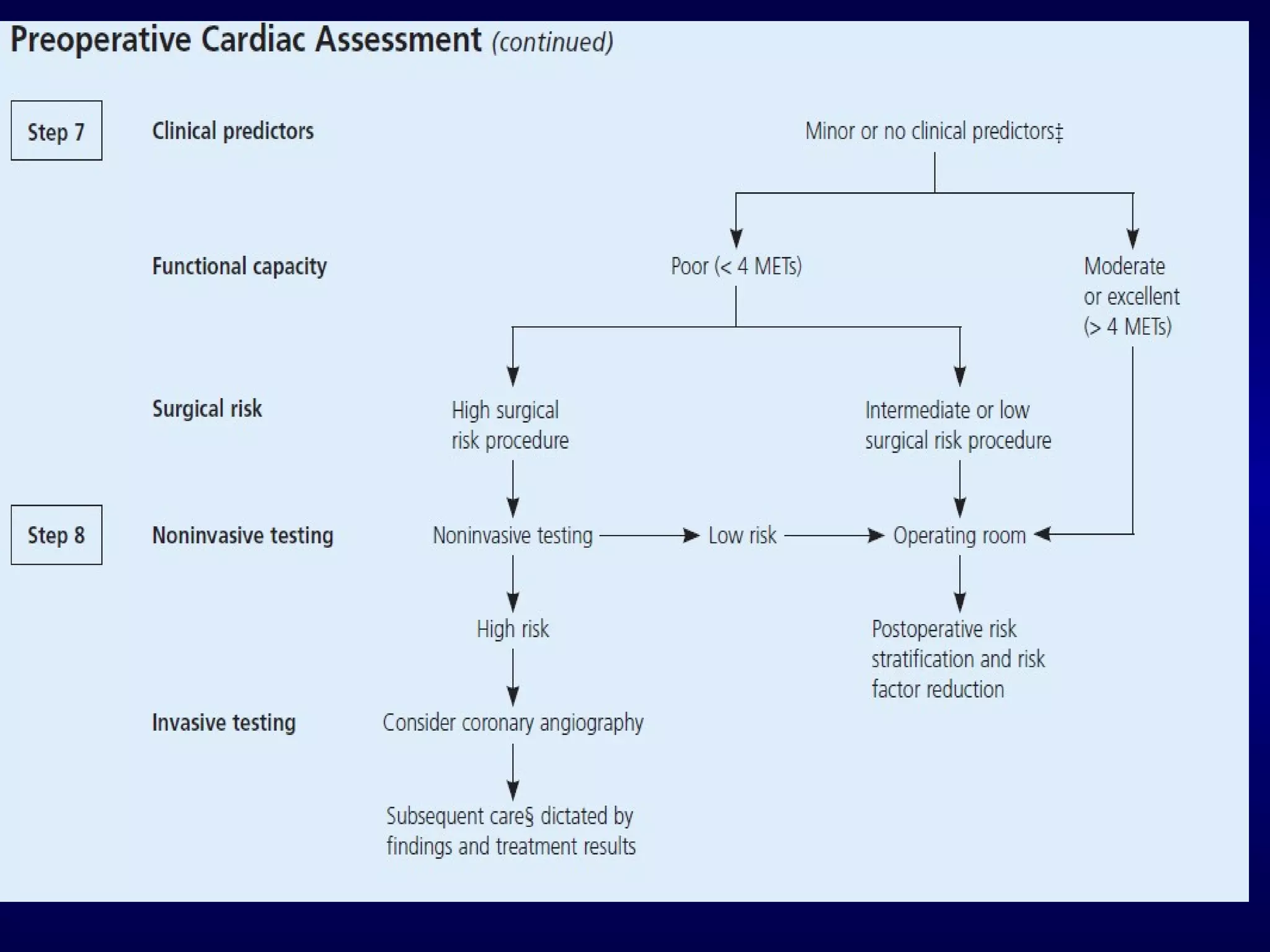
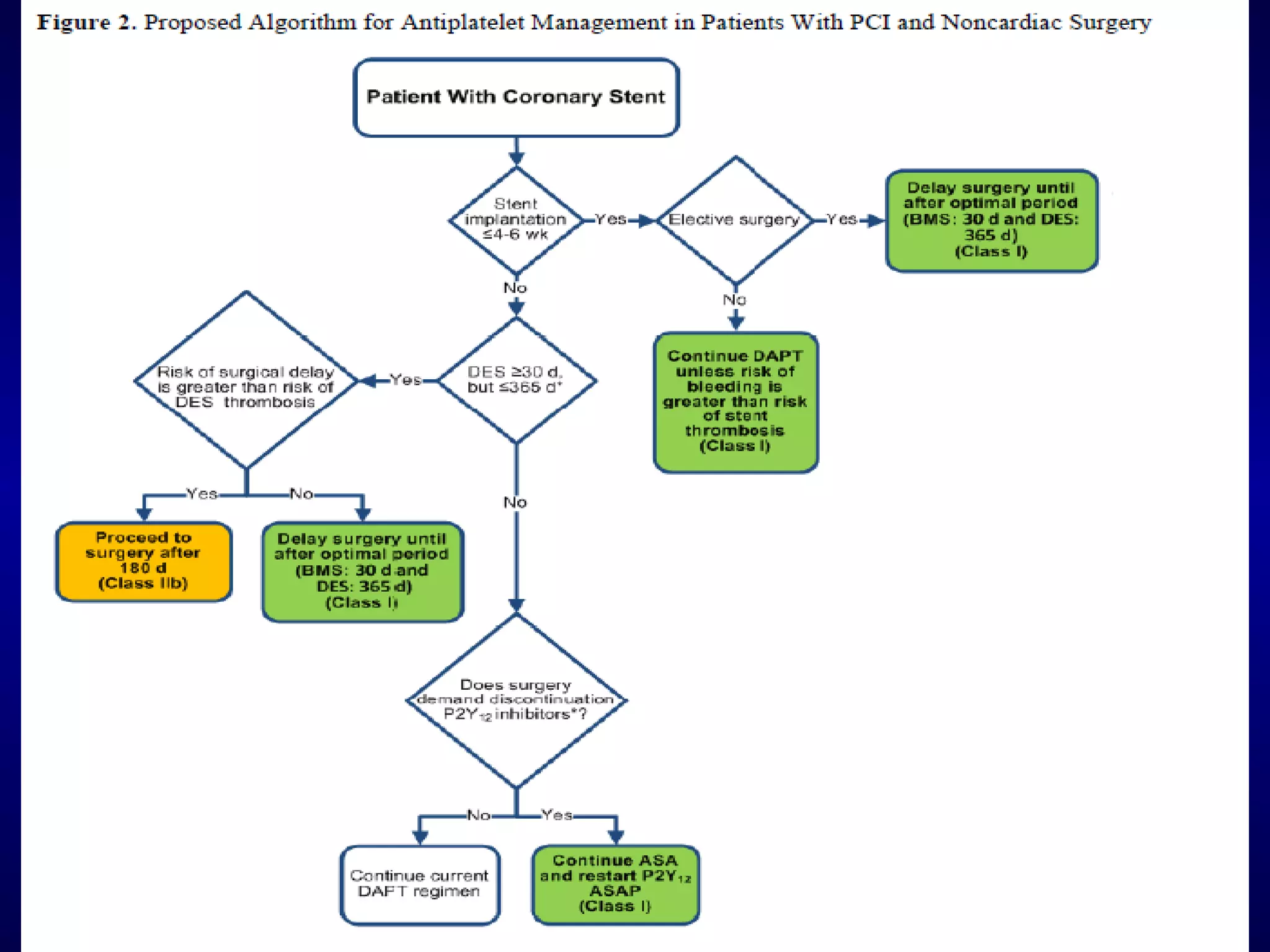
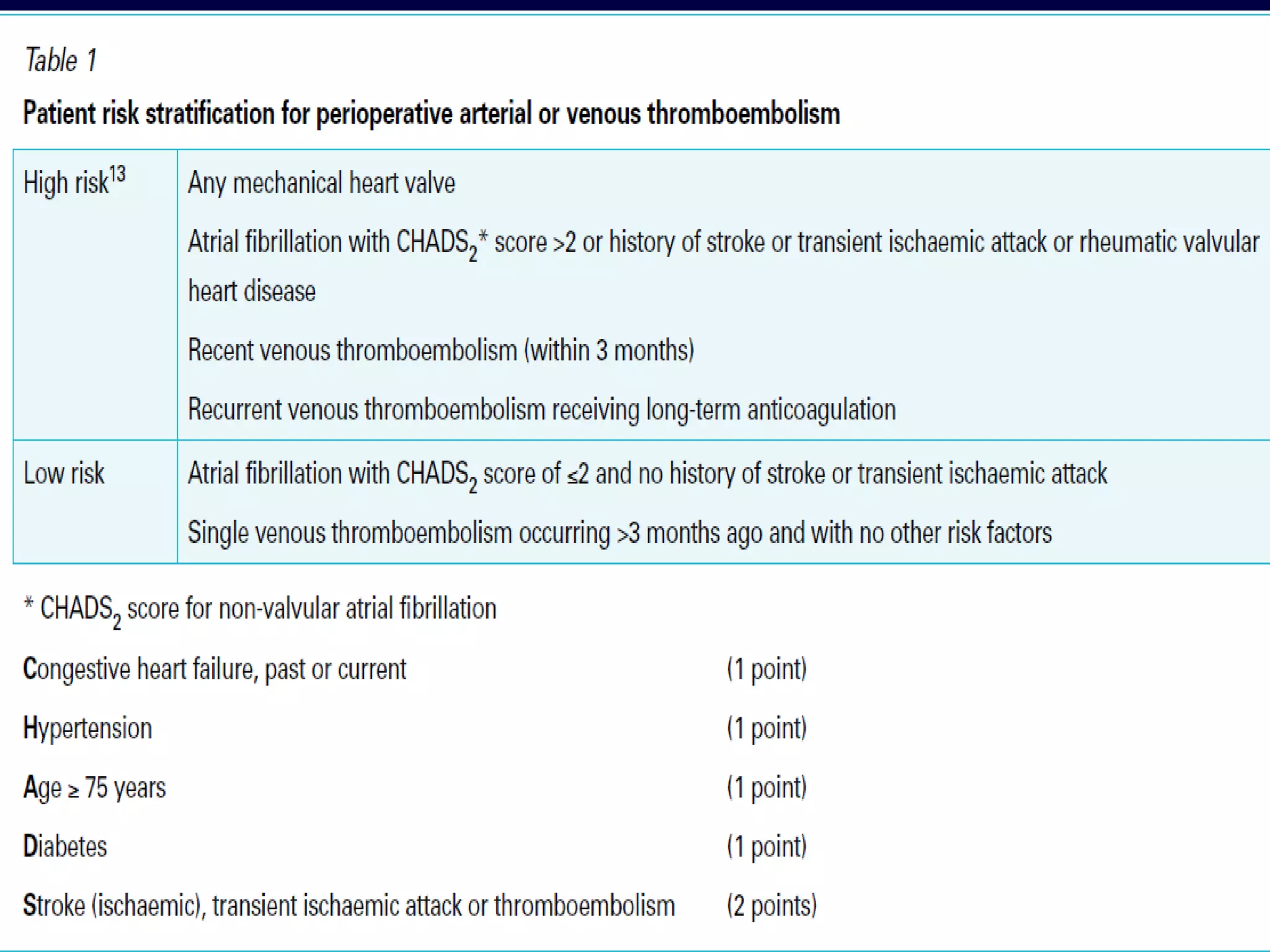
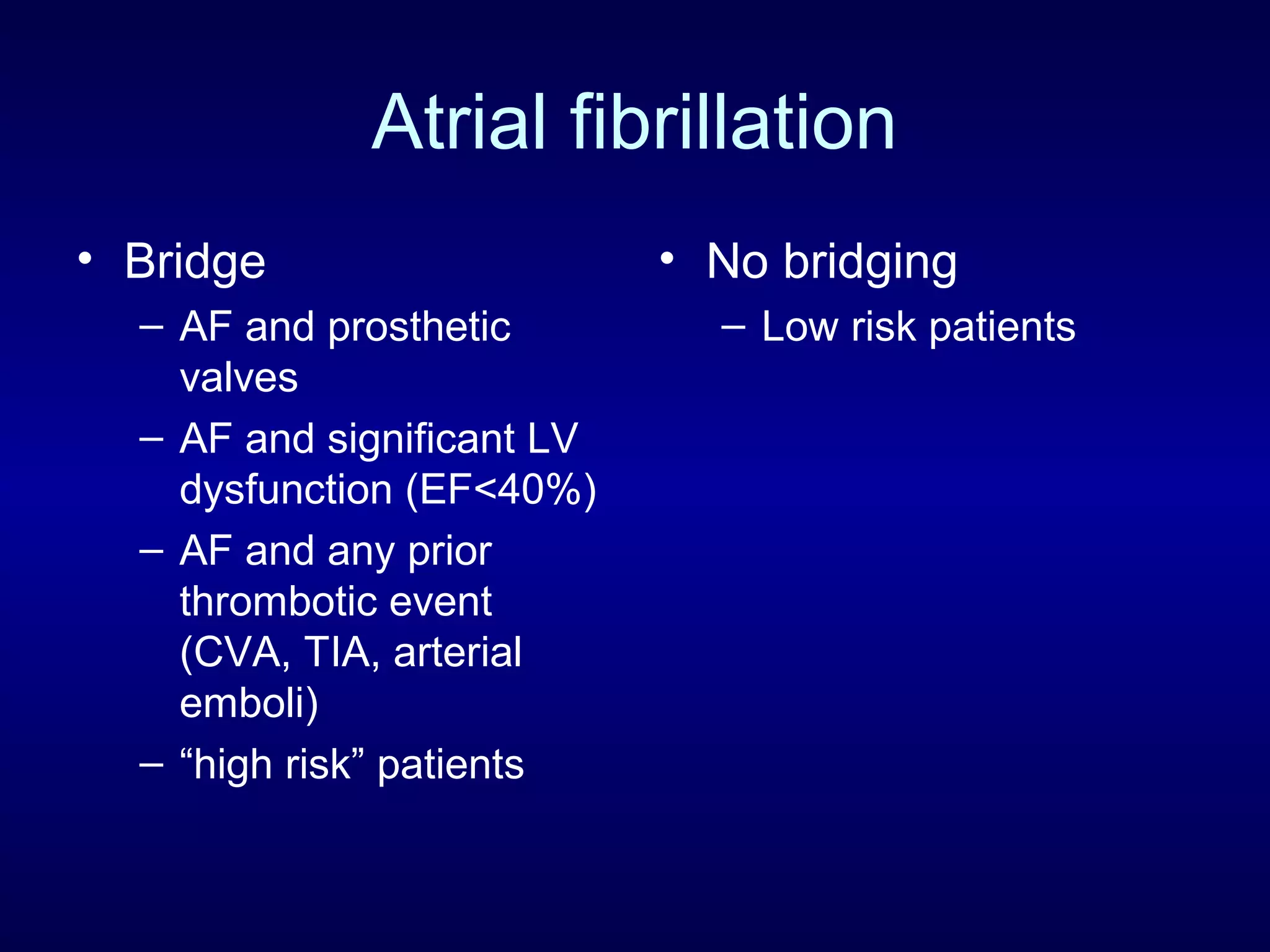
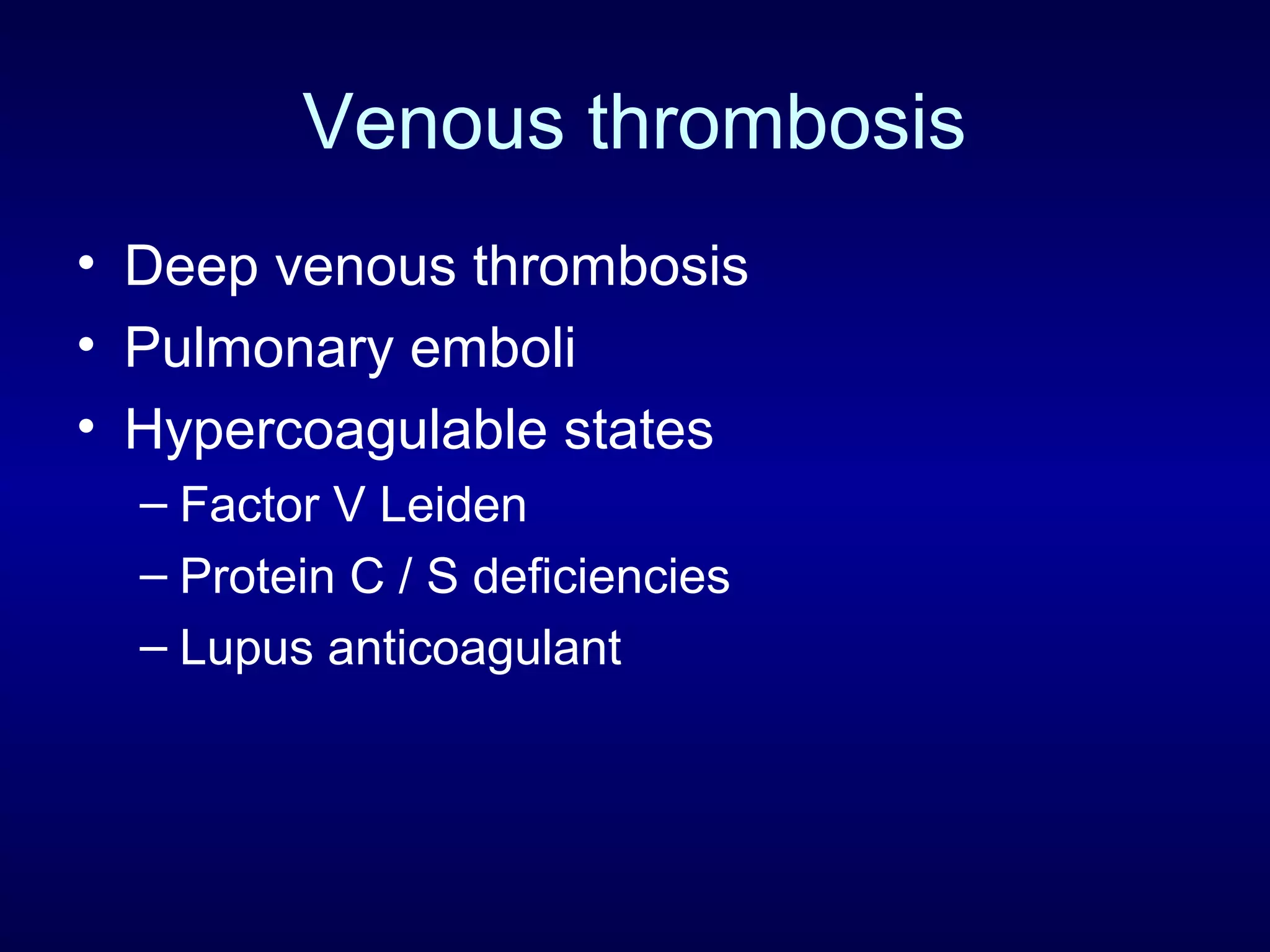
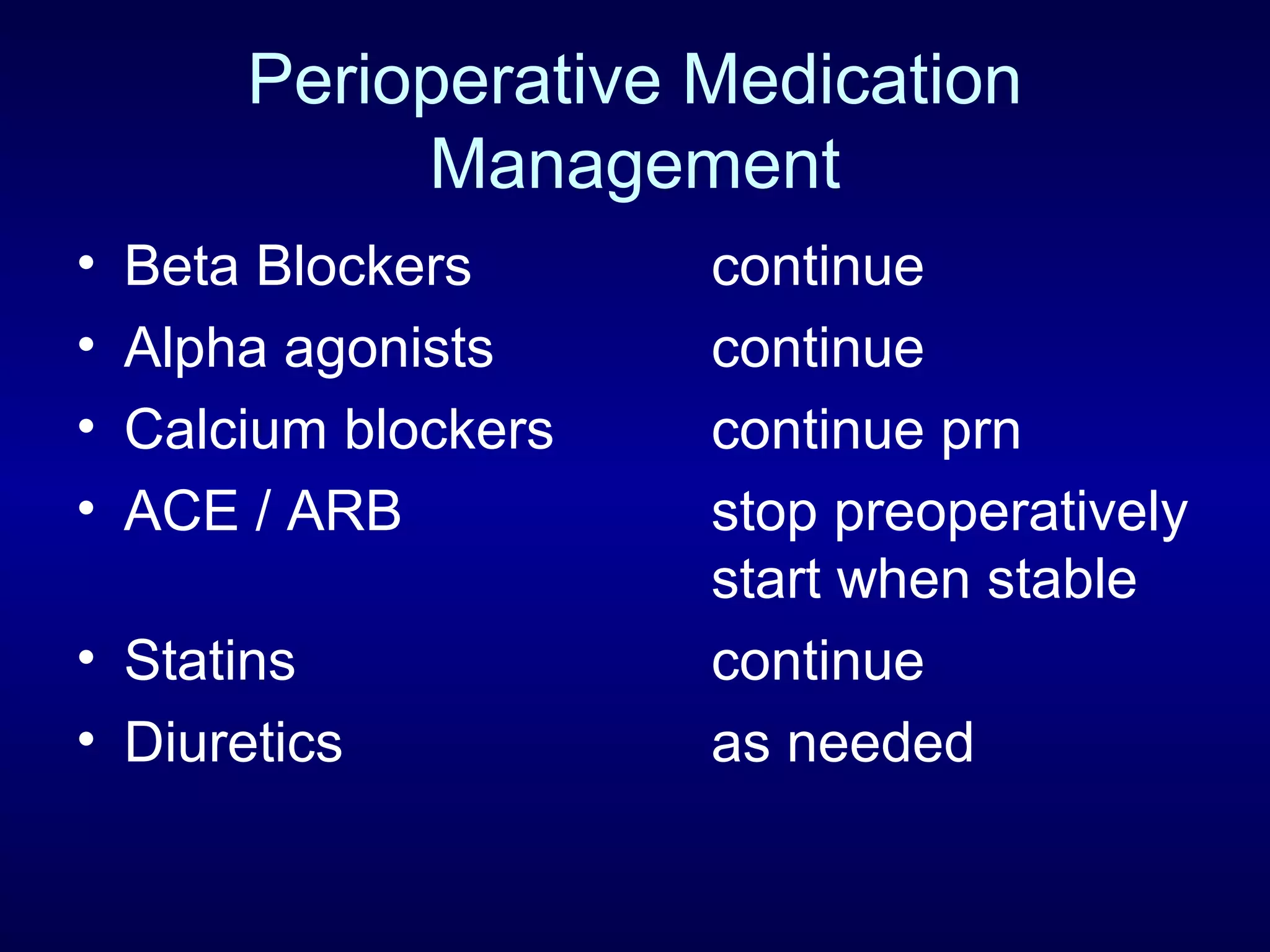
This document discusses cardiac issues related to non-cardiac surgery and the role of antiplatelet and anticoagulant medications in the perioperative period. It notes that the aging population has increased coronary artery disease prevalence and antiplatelet agents are widely prescribed afterwards. It also discusses factors that increase stent thrombosis risk and the importance of continuing dual antiplatelet therapy. The document covers preoperative risk assessment, medication management of antiplatelets and anticoagulants in the perioperative period, and postoperative management strategies to reduce cardiac complications of non-cardiac surgery.