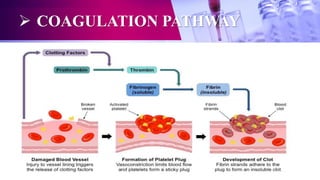
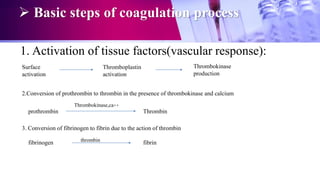
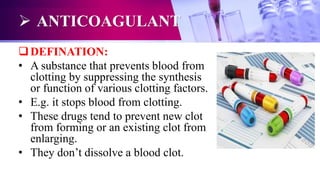
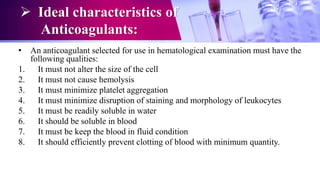
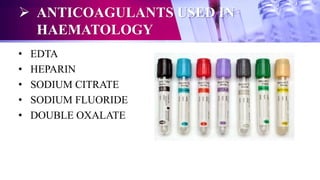
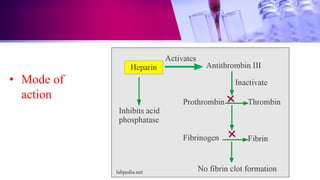
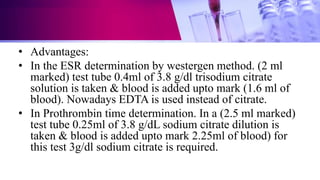
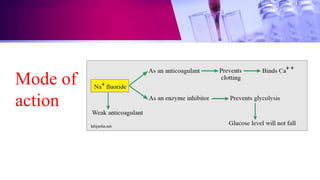
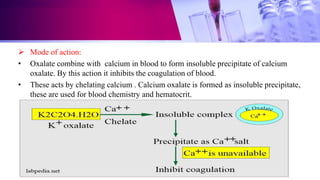
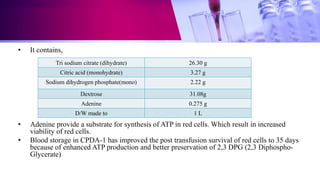
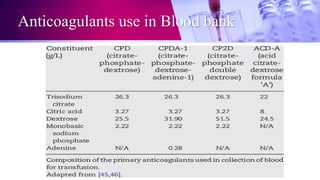
This document discusses anticoagulants used in hematology and blood banking. It begins by describing the coagulation pathway and defining anticoagulants. It then discusses various anticoagulants used in hematology like EDTA, heparin, sodium citrate, sodium fluoride, and double oxalate. It notes their ideal characteristics, modes of action, concentrations used, advantages, and disadvantages. The document concludes by outlining anticoagulant solutions used in blood banking like ACD, CPD, CPDA-1, and CPDA-2. It provides the constituents of each solution and how they improve storage of red blood cells and post-transfusion viability.