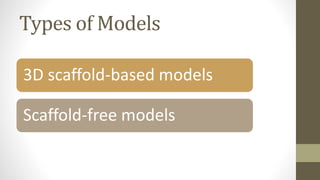
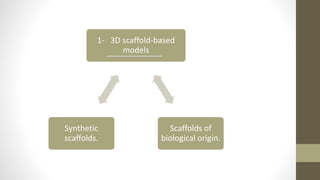
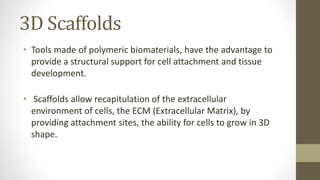
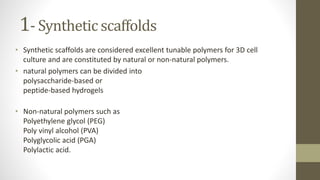
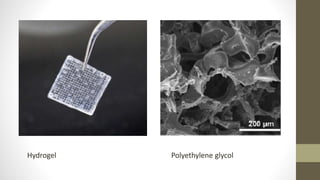
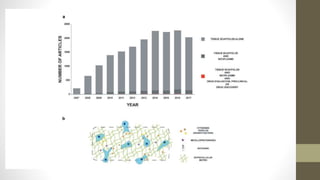
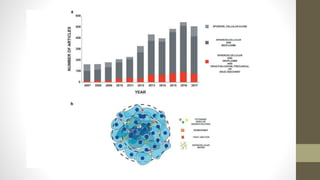
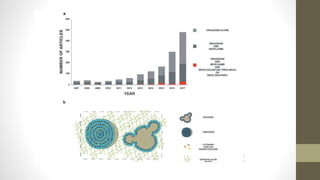
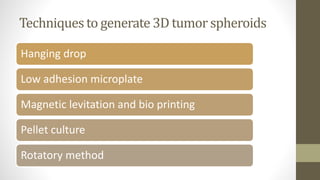
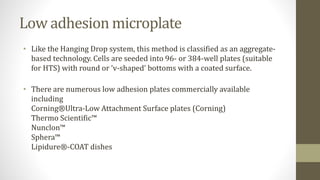
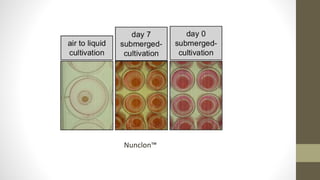
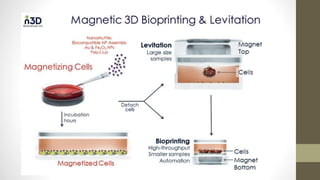
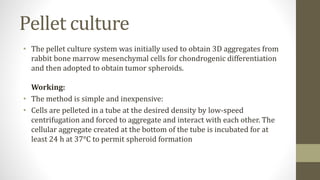
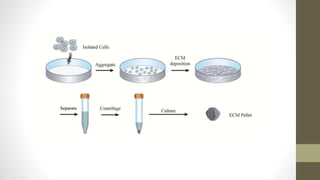
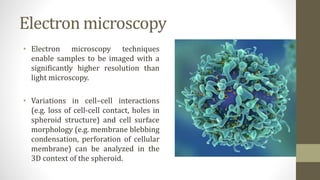
The document discusses anticancer drug discovery utilizing multicellular tumor spheroid models, outlining types of models, techniques for spheroid generation, and readout strategies for analysis. It emphasizes the advantages and disadvantages of both scaffold-based and scaffold-free models, particularly highlighting the complex interactions within spheroids that can affect drug response. The conclusion warns about the limitations of these models in accurately predicting clinical outcomes due to variability and lack of standardized protocols.