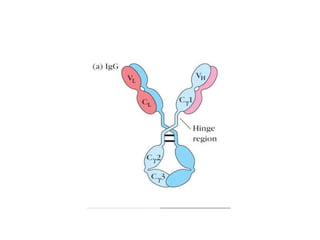
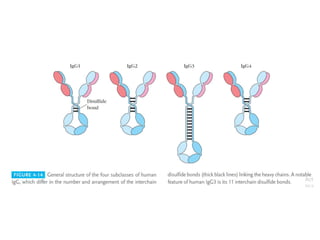
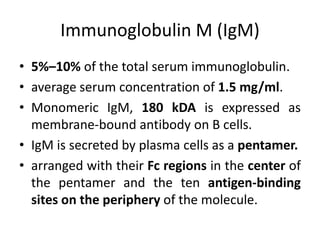
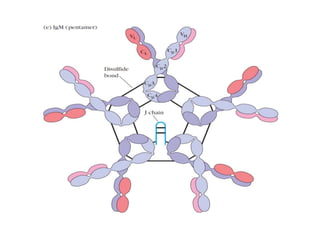
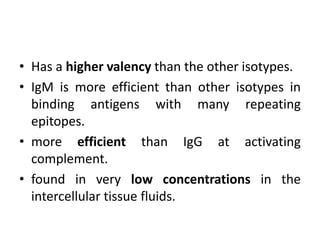
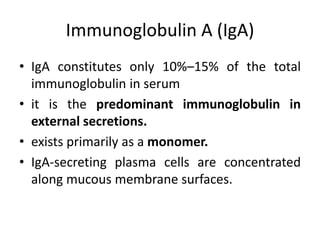
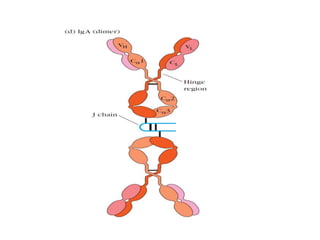
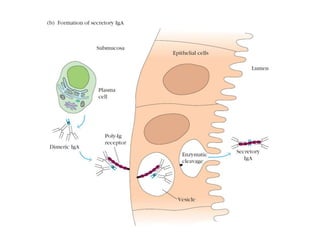
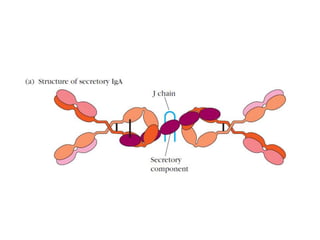
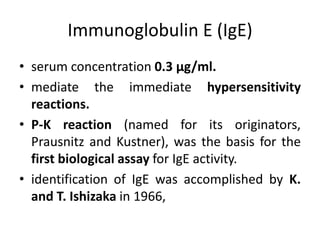
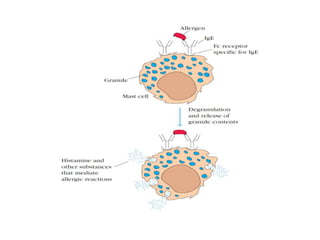
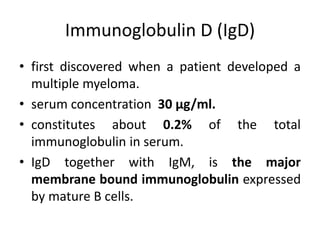
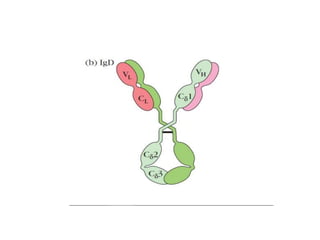
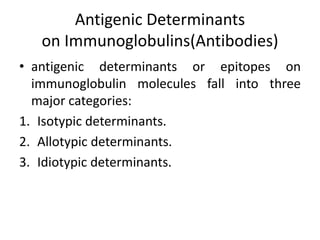
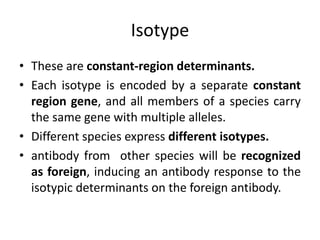
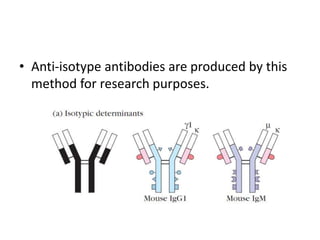
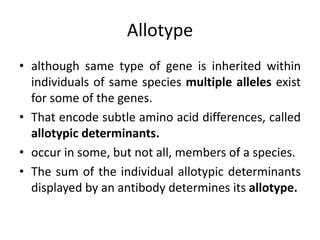
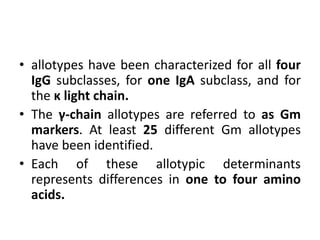
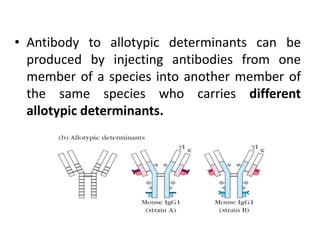
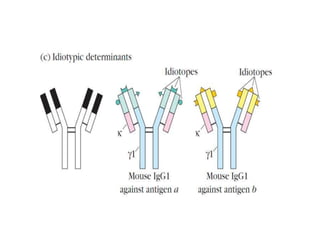
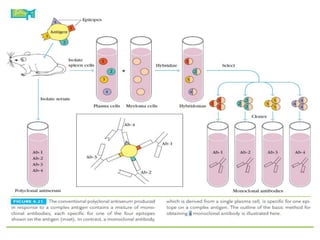
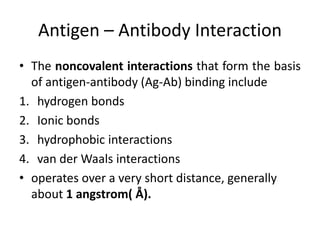
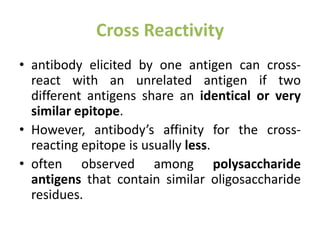
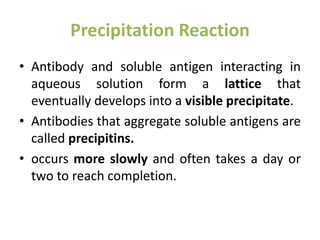
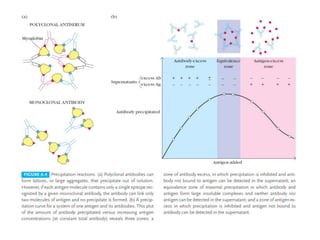
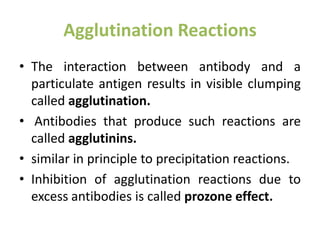
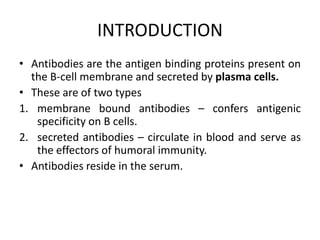
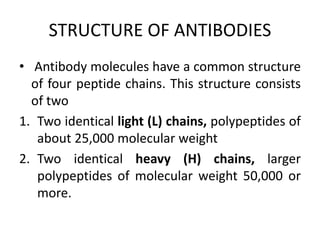
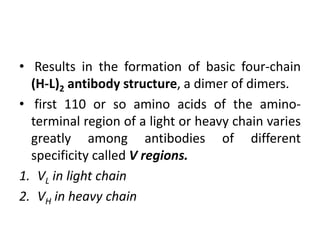
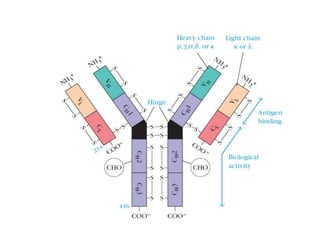
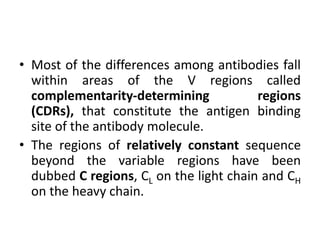
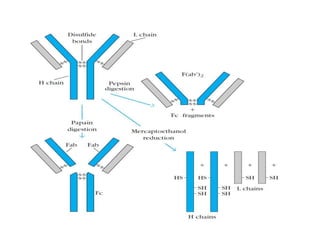
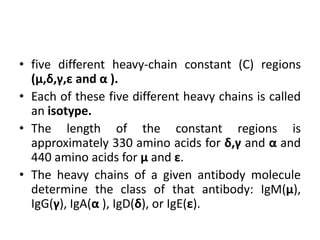
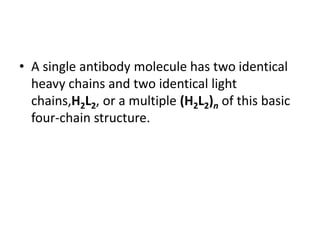
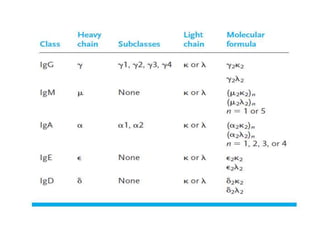
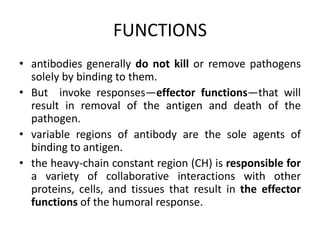
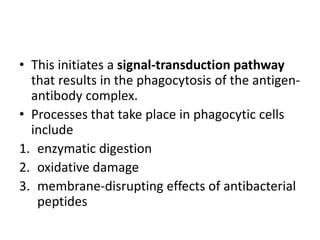
The document discusses the structure, classes, and functions of antibodies. It begins by describing the basic four-chain structure of antibodies consisting of two heavy chains and two light chains. It then discusses the five classes of antibodies - IgG, IgM, IgA, IgE, and IgD - and their properties such as structure, location, and roles in immune responses. The document also covers antigen binding regions, monoclonal antibodies, antigen-antibody interactions, and cross-reactivity.






![3. ANTIBODY DEPENDENT CELL
MEDIATED CYTOTOXICITY[ADCC]
• The linking of antibody bound to target cells
(virus infected cells of the host) with the Fc
receptors of a number of cell types,
particularly natural killer (NK) cells, can direct
the cytotoxic activities of effector cell on the
target cell.
• The antibody acts as a newly acquired
receptor enabling the attacking cell to
recognize and kill the target cell.](https://image.slidesharecdn.com/antibodiesi-200109131703/85/Antibodies-classes-and-function-19-320.jpg)