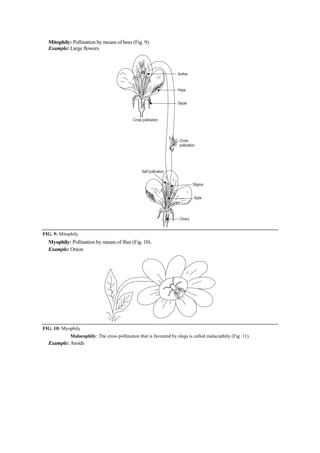
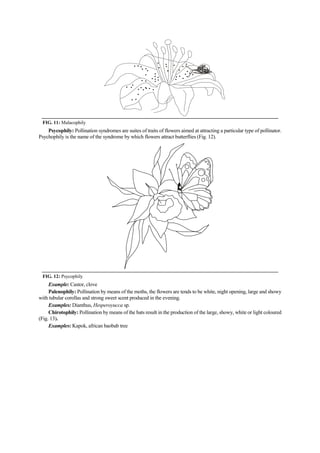
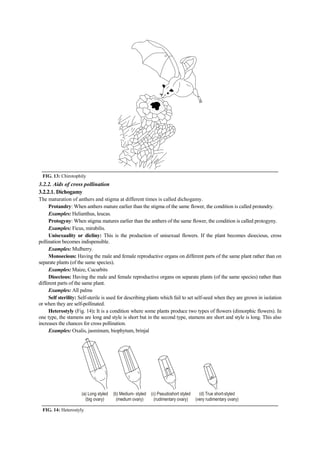
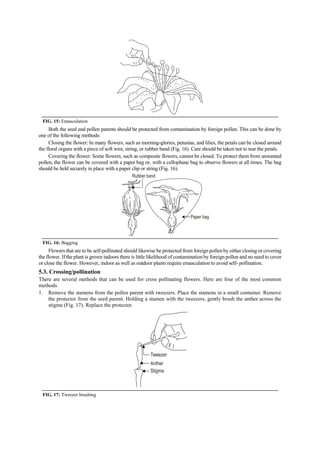
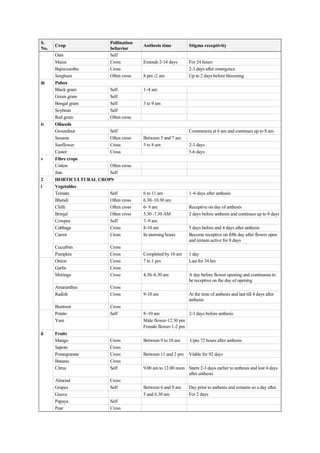
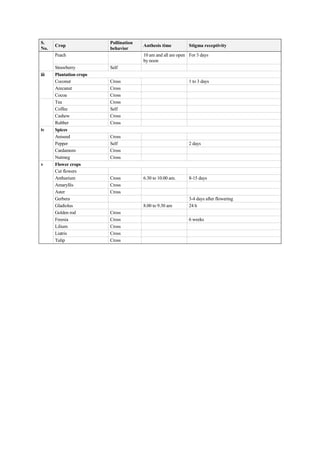
The document discusses the processes of anthesis and pollination in flowering plants, highlighting the stages of flowering and the mechanisms of both self and cross-pollination. It explores various types of pollination strategies, including mechanisms like dichogamy, cleistogamy, and adaptations for different pollinators such as insects, wind, and water. Additionally, the document outlines the steps involved in pollination, including prepollination and emasculation to ensure successful fertilization.