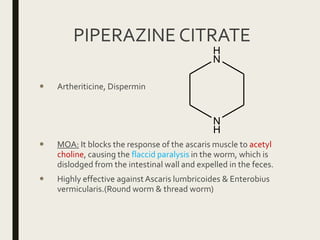
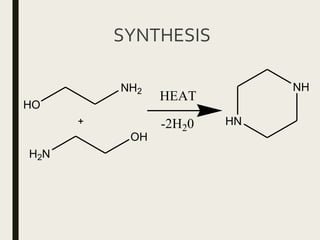
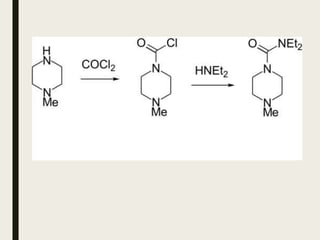
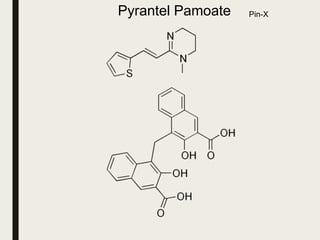
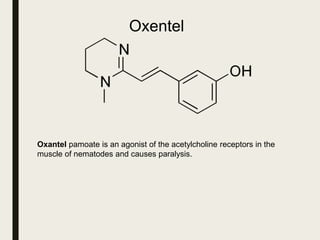
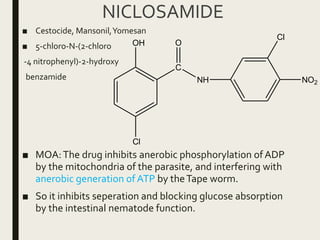
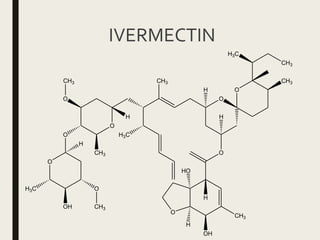
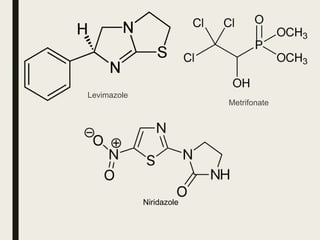
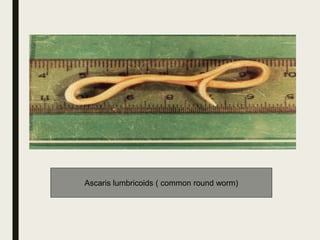
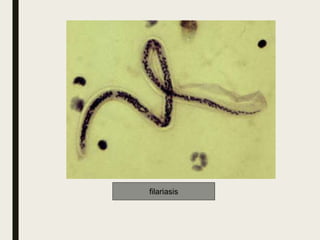
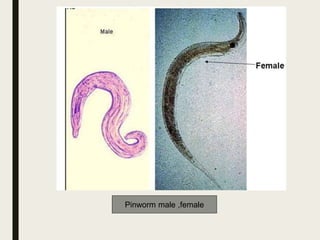
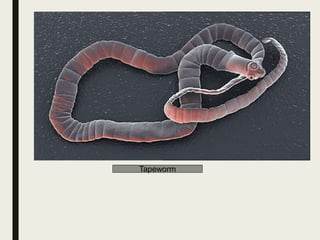
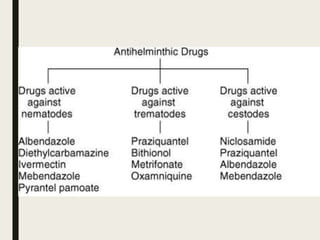
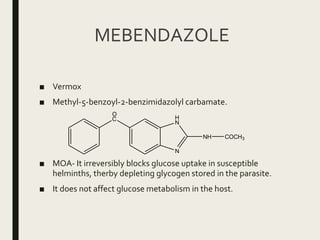
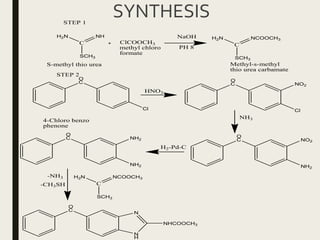
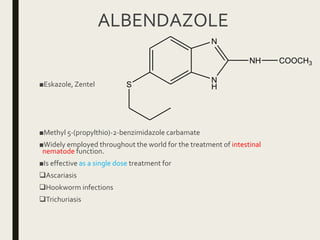
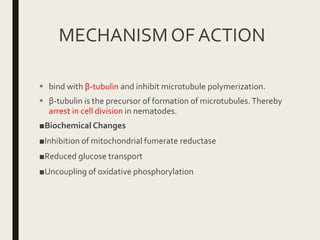
This document defines anthelmintics as agents used to destroy or eliminate parasitic worms from the gastrointestinal tract. It classifies anthelmintics and discusses several common drugs used as anthelmintics including mebendazole, albendazole, thiabendazole, oxaminiquine, praziquantel, piperazine citrate, diethylcarbamazine citrate, pyrantel pamoate, oxentel, niclosamide, and ivermectin. The document provides details on the chemical structure, mechanism of action, and uses of many of these anthelmintic drugs.











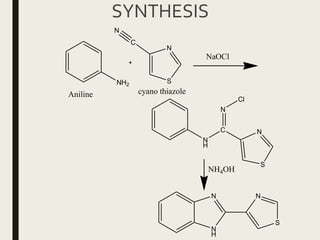
![THIABENDAZOLE
Mintezol
2[4-thiazolyl]benzimidazole
N
H
N
S
N](https://image.slidesharecdn.com/anthelmintics-191021050116/85/Anthelmintics-20-320.jpg)

![OXAMNIQUINE
■ Vansil
■ 1,2,3,4-tetrahydro-2-[isopropyl-amino) methyl]-7-nitro-6-
quinoline methanol.
■ It inhibits DNA, RNA & protein synthesis.
■ SAR- OH group is essential for activity.
■ For the treatment of intestinal schistosomiasis.
N
H
O2N
HOH2C
CH2NHCH](https://image.slidesharecdn.com/anthelmintics-191021050116/85/Anthelmintics-24-320.jpg)
![PRAZIQUANTEL
■ Biltricide
■ 2-(cyclohexyl carbonyl)-1,2,3,6,7,11b-hexahydro-4H-
pyrazino[2,1-a]isoquinolin-4-one.
■ It increases cell membrane permeability of susceptible worms,
resulting in loss of intracellular calcium and loss of extracellular
sodium.
N
N
C
O](https://image.slidesharecdn.com/anthelmintics-191021050116/85/Anthelmintics-25-320.jpg)