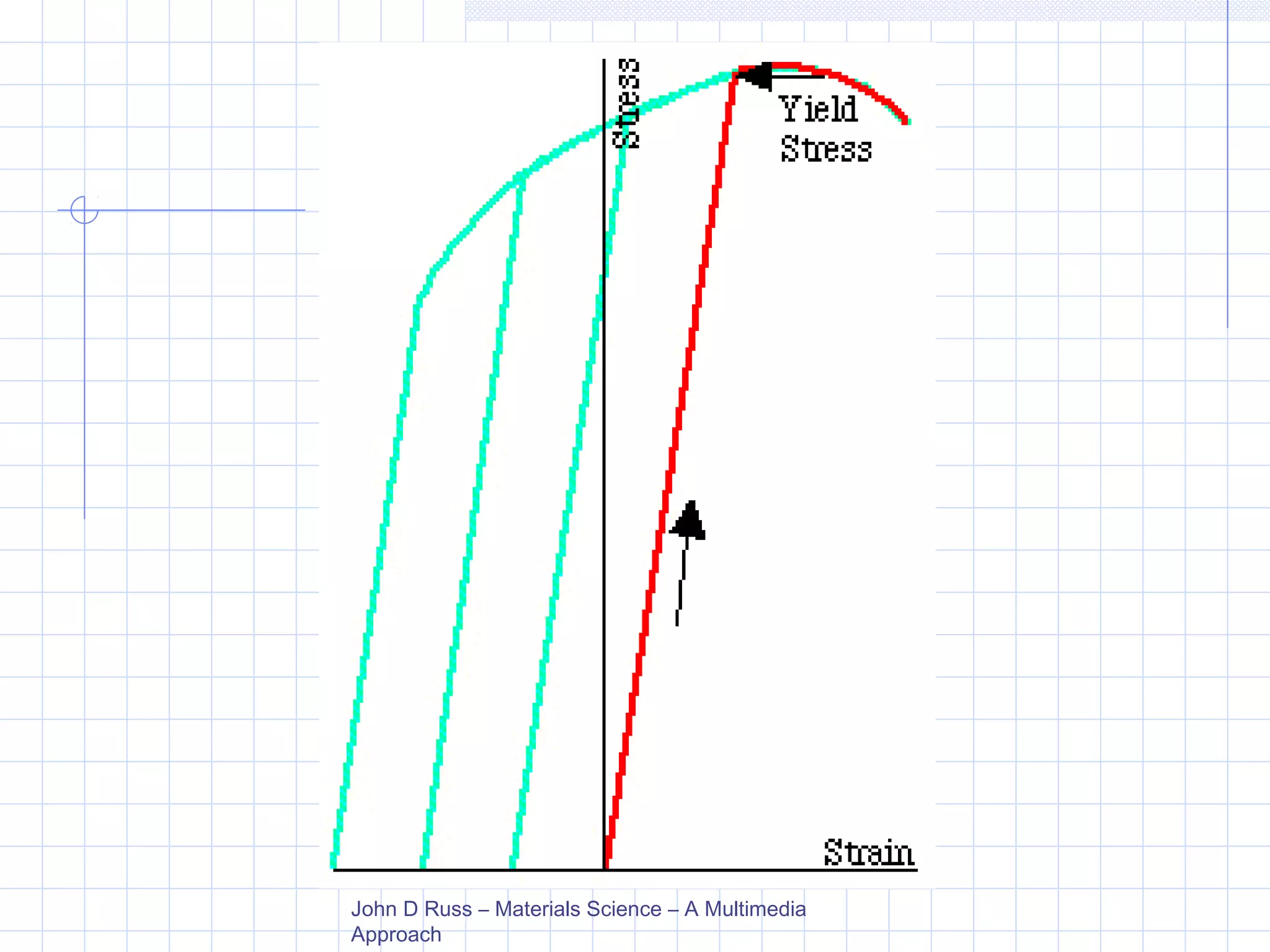
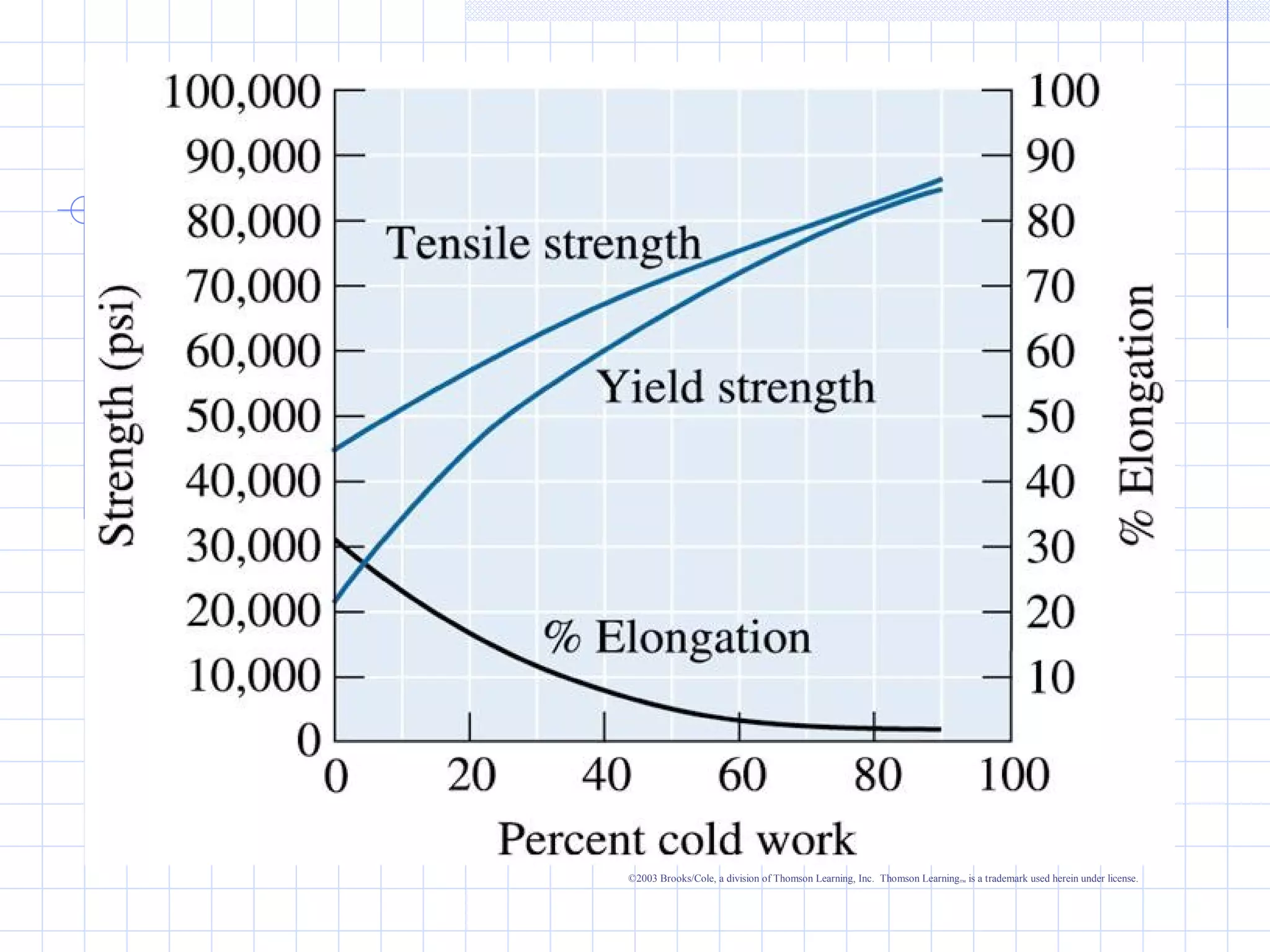
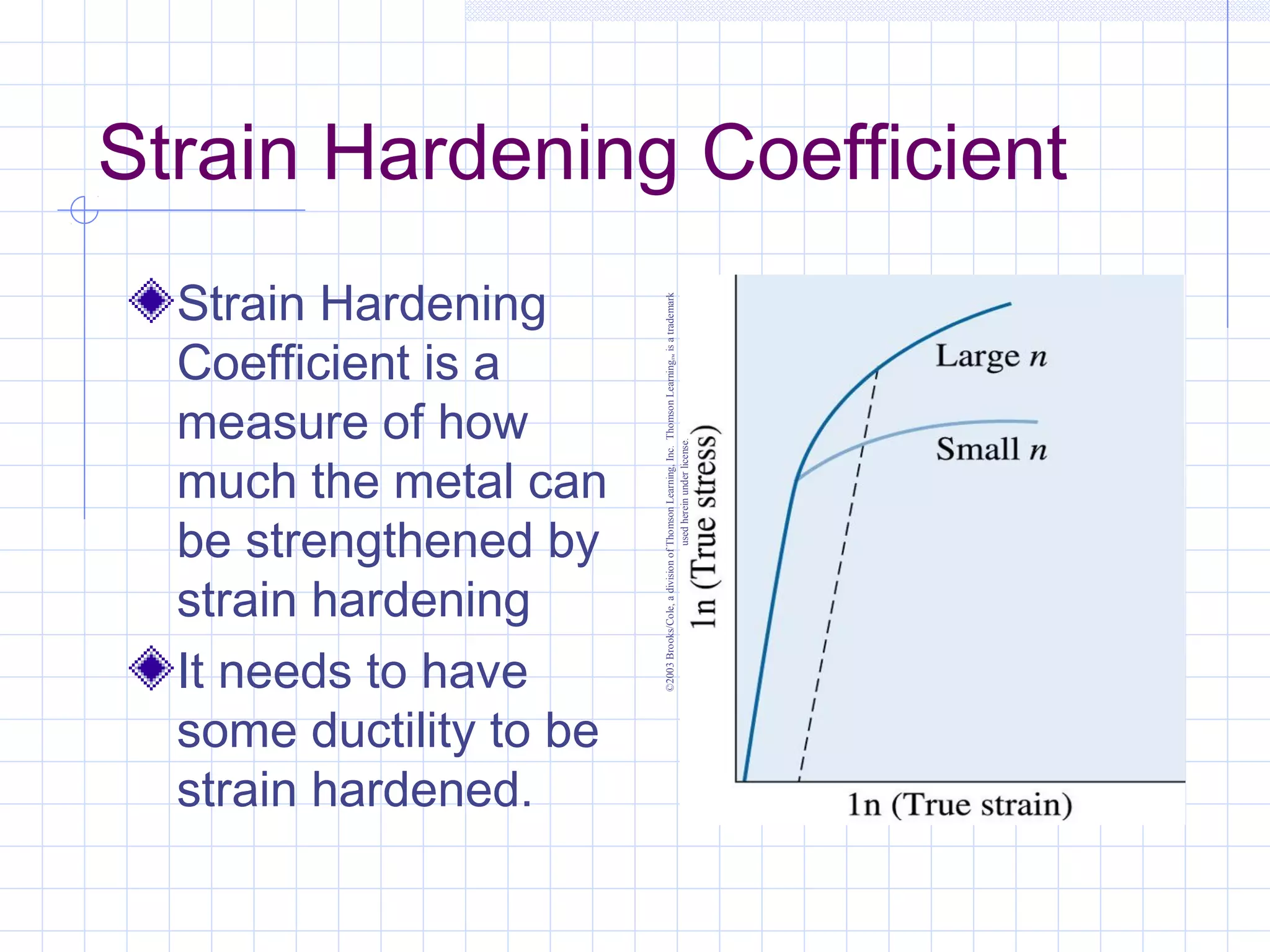
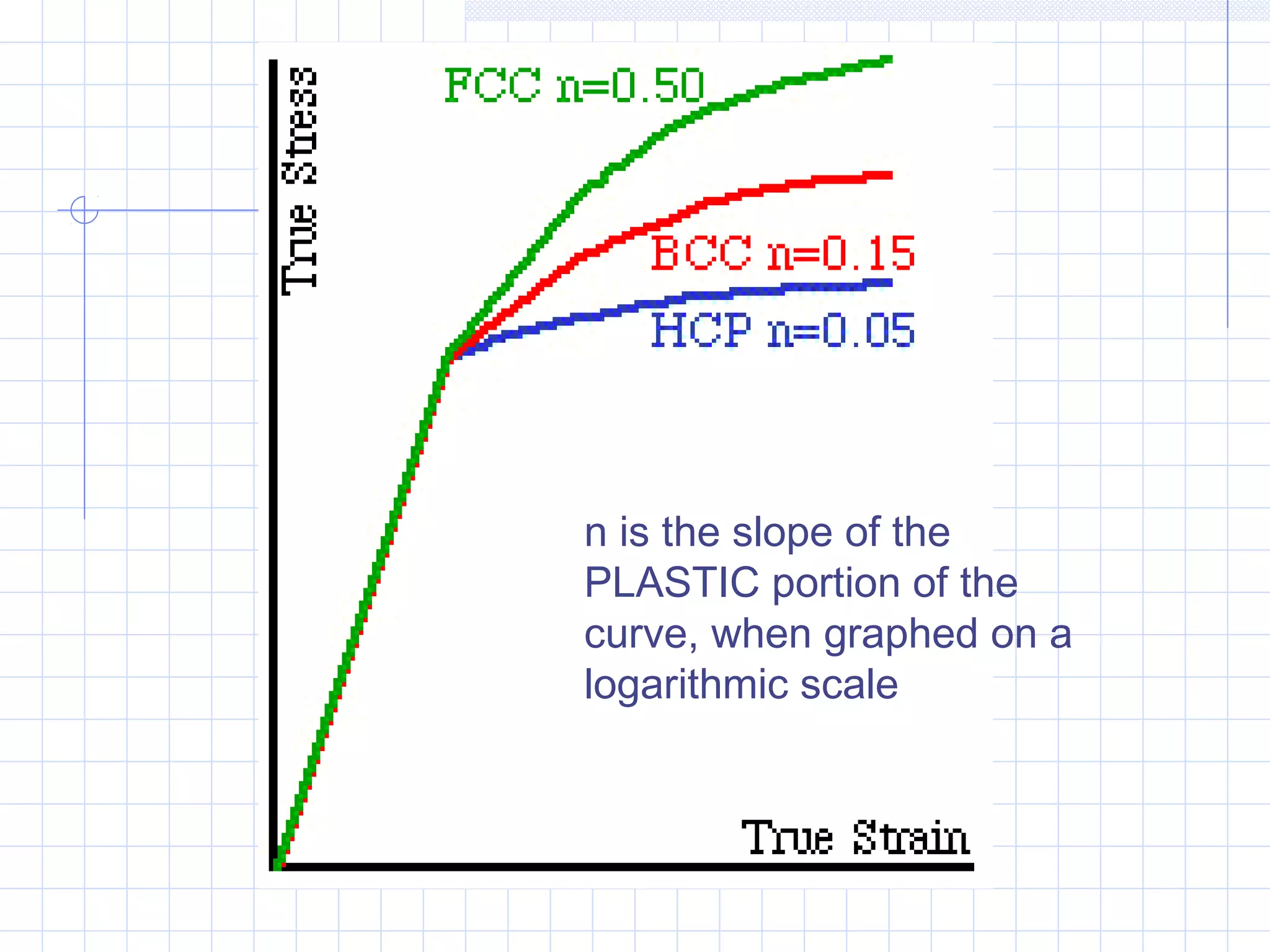
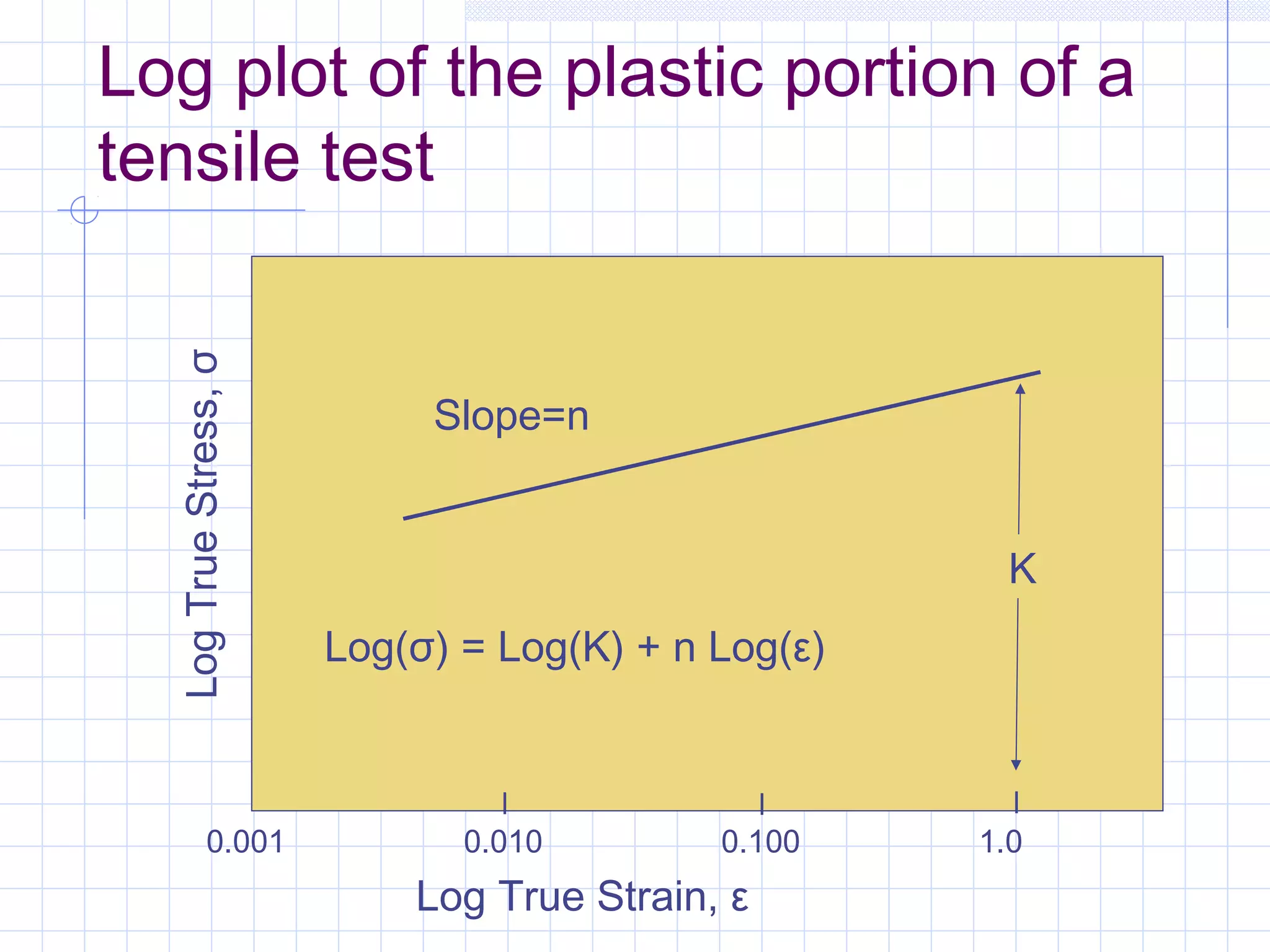
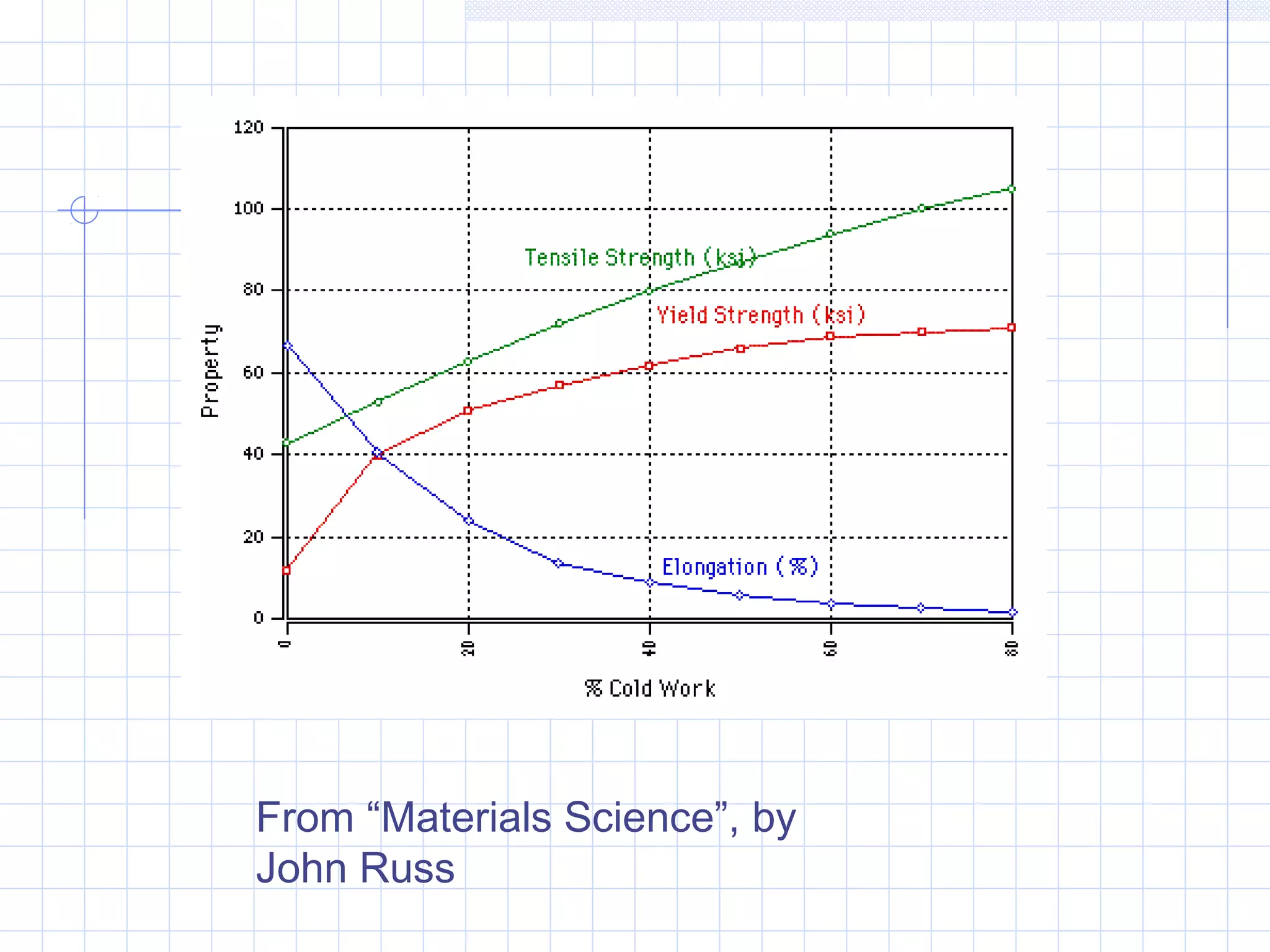
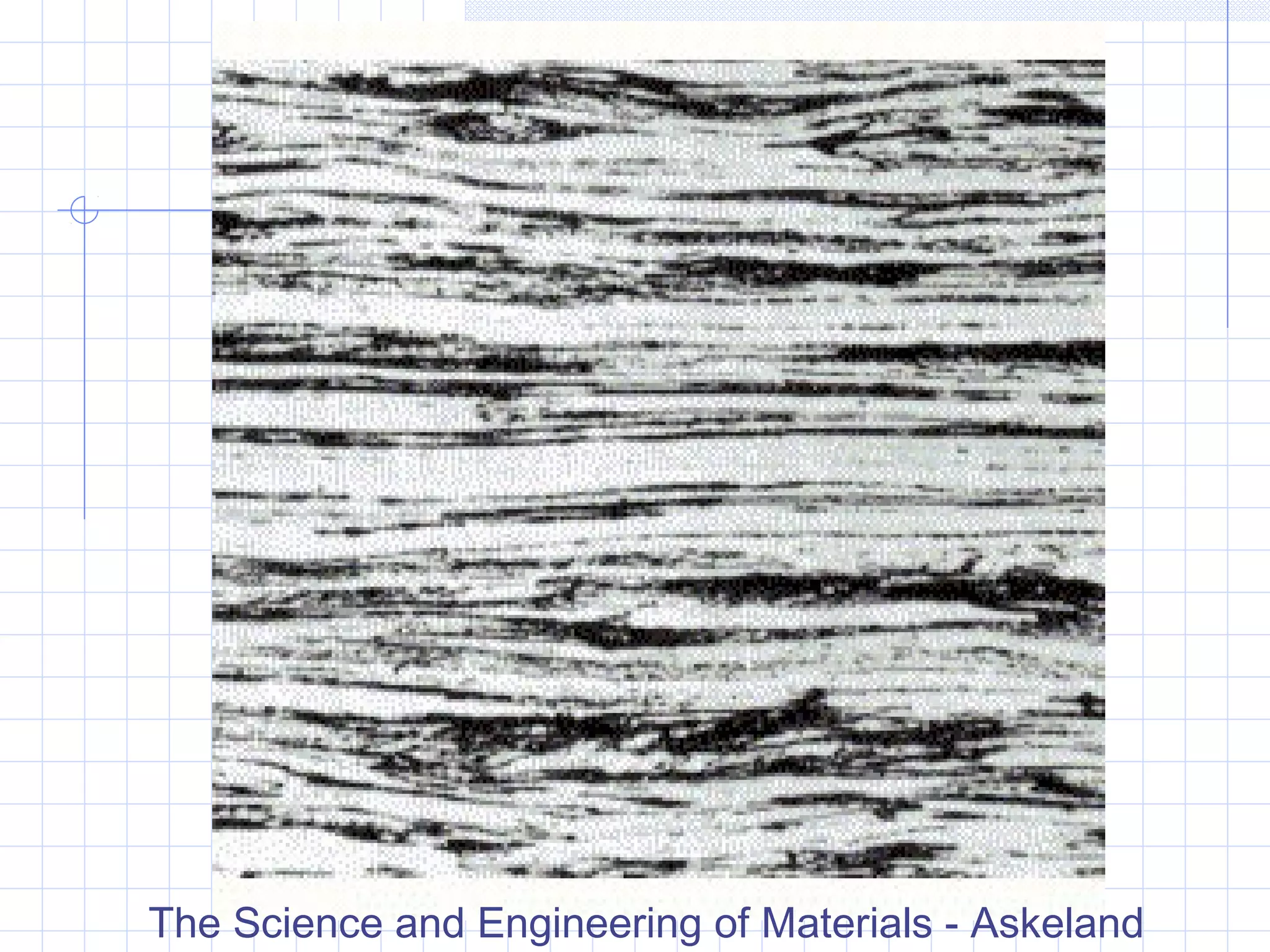
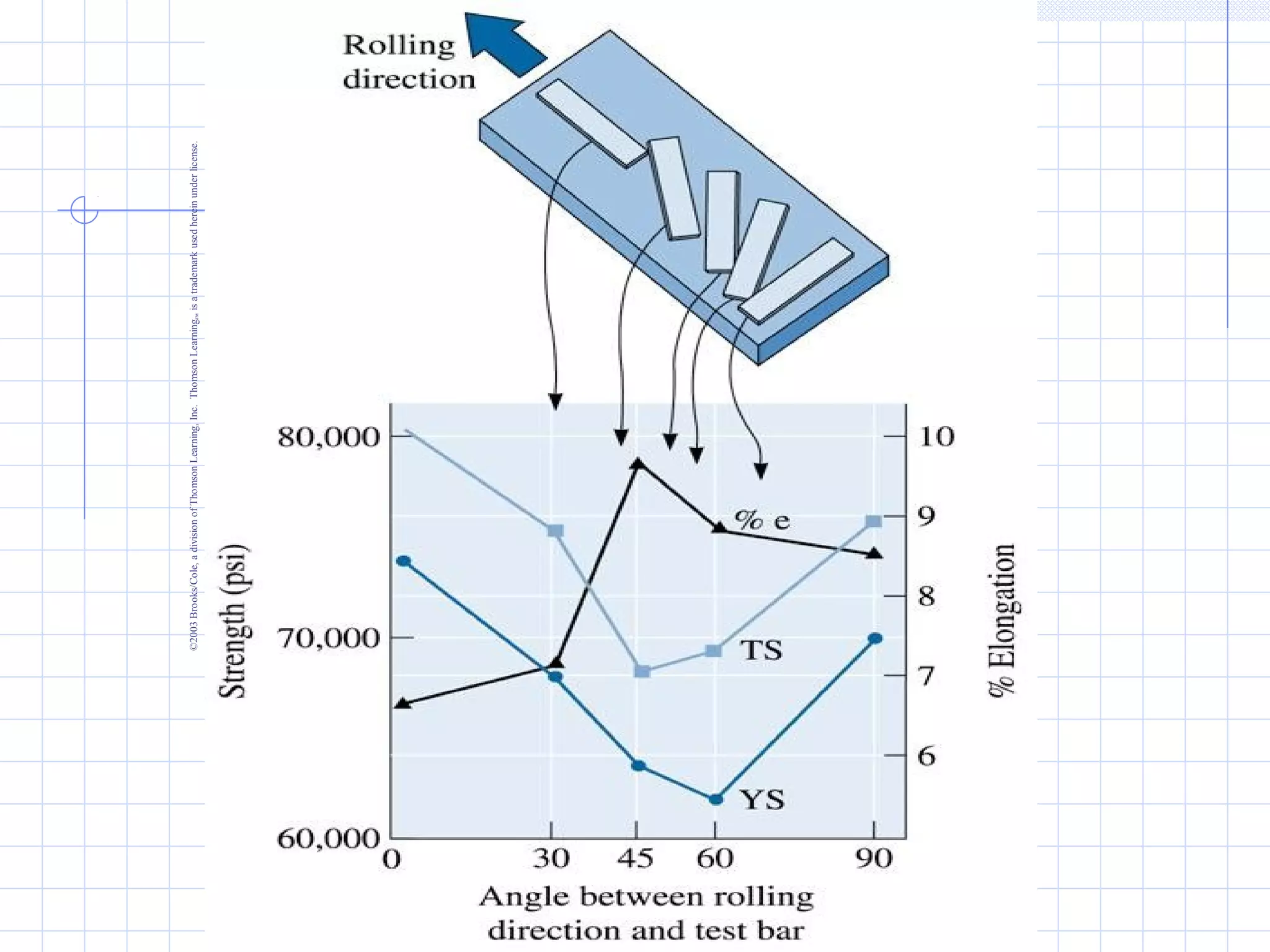
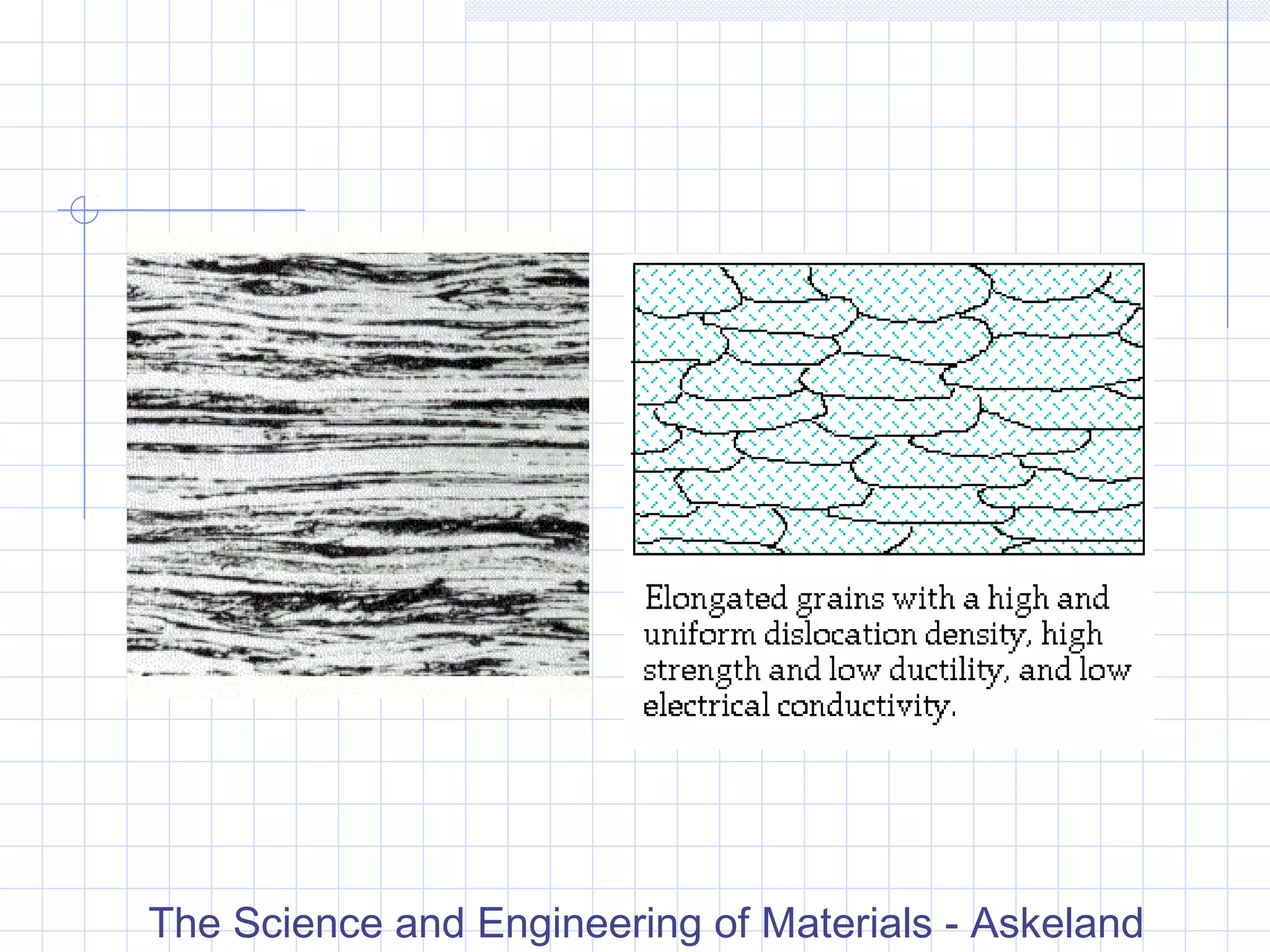
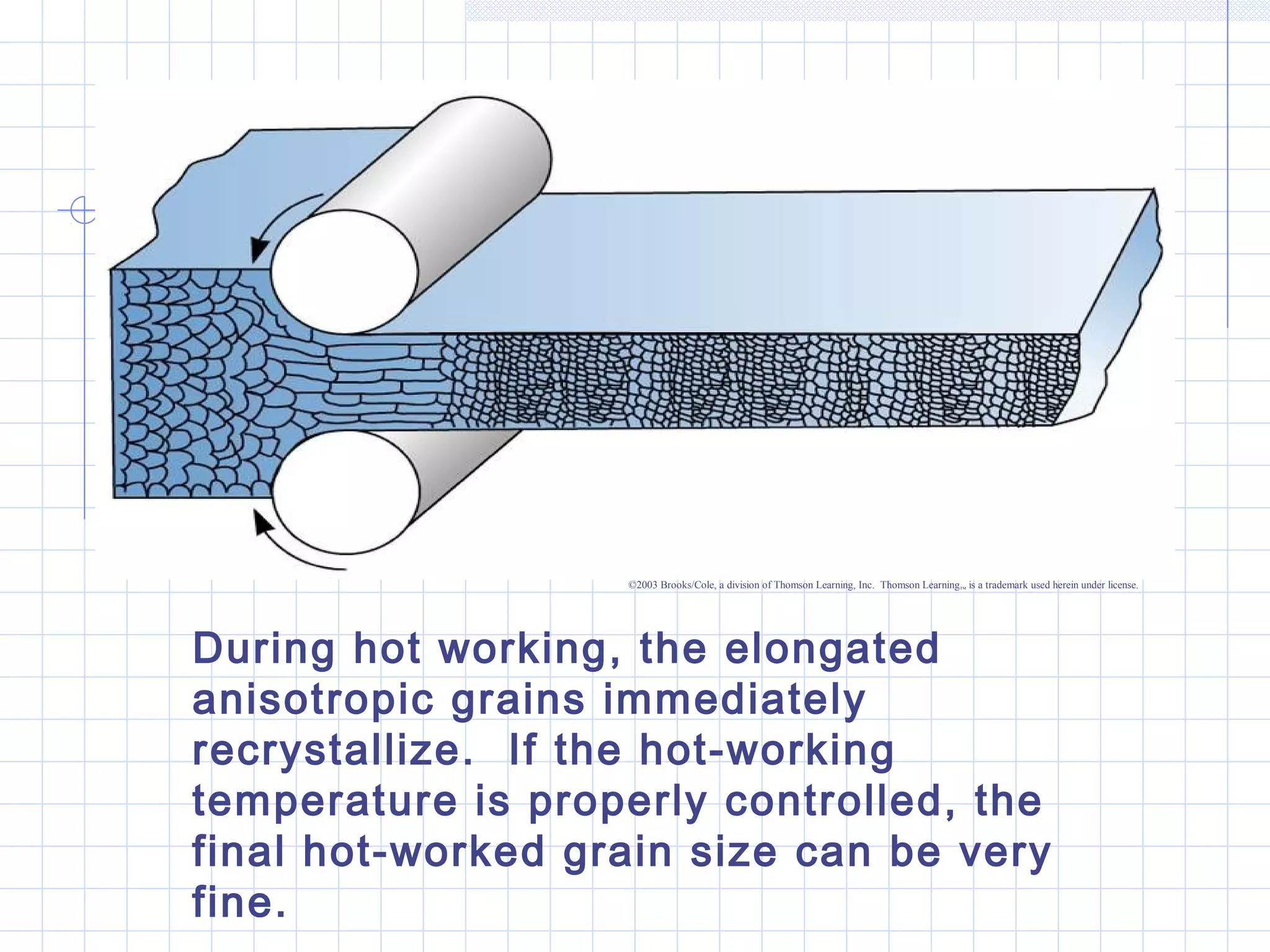
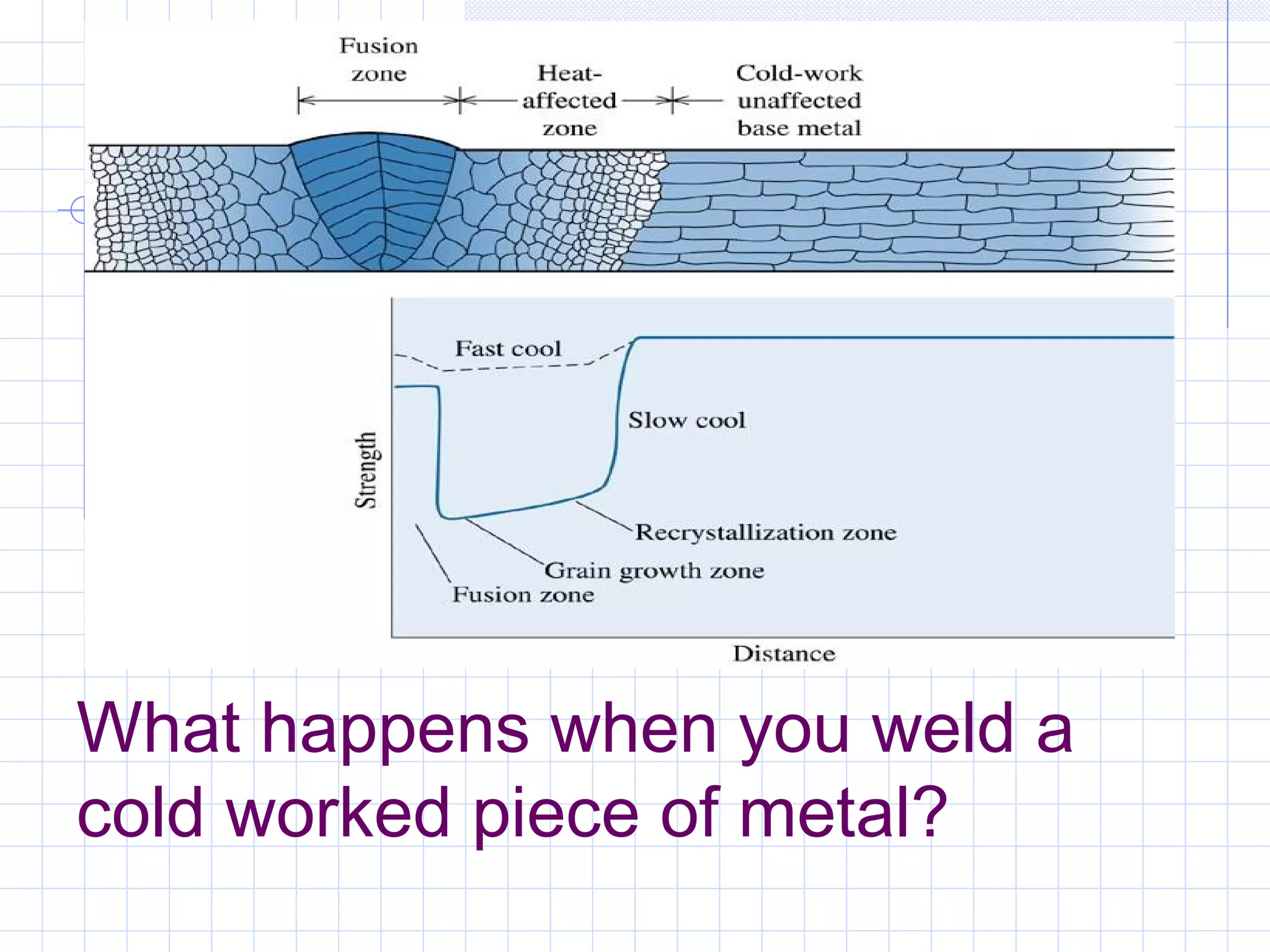
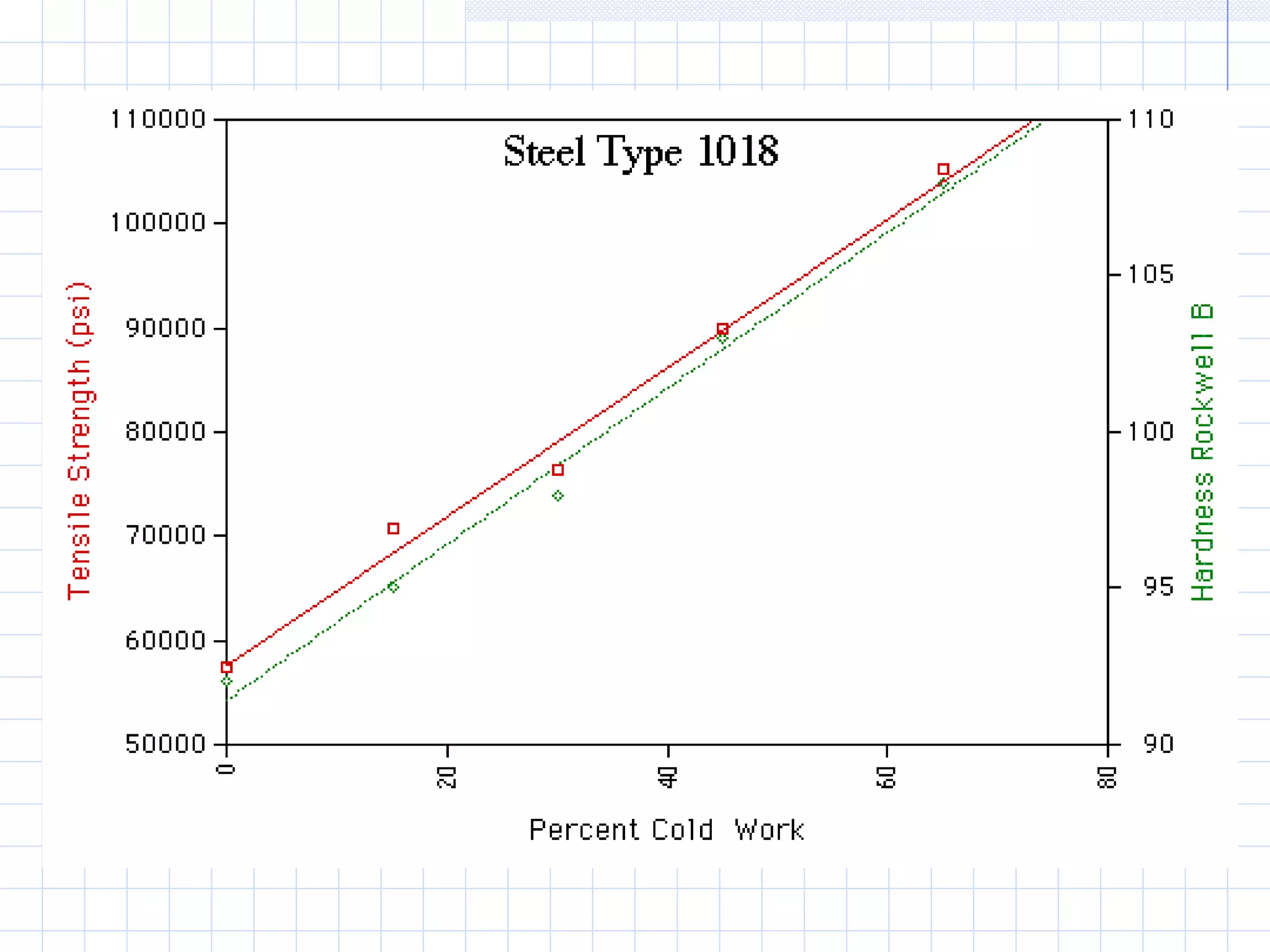
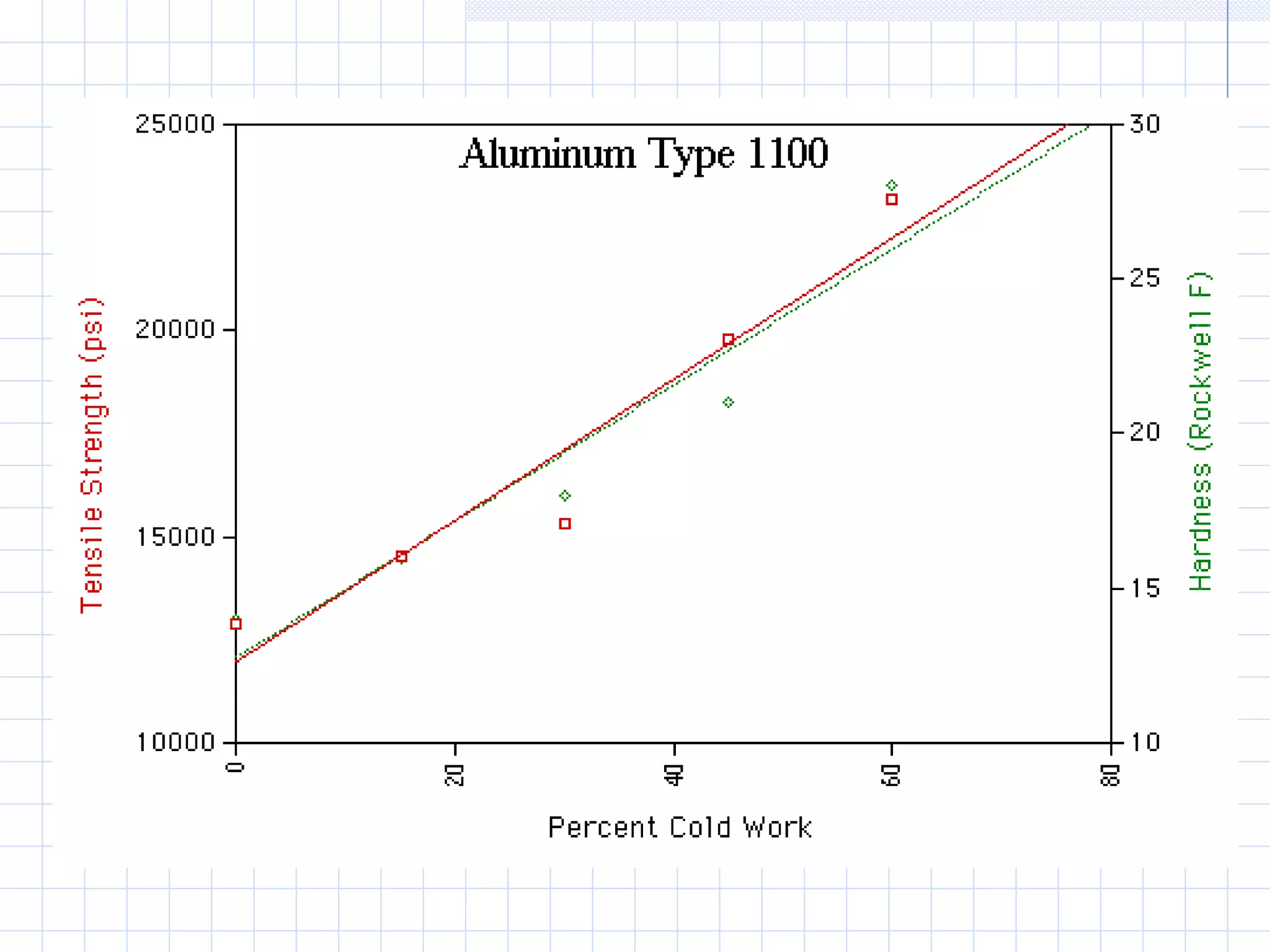
Strain hardening occurs when dislocations in a deformed metal interact, increasing the material's strength. Deforming a metal increases the number of dislocations, further strengthening the material. Strain hardening is measured by properties like yield strength and tensile strength increasing while ductility decreases. The material becomes harder but more brittle. Annealing can be used to "undo" strain hardening by allowing dislocations to rearrange or new grains to form, restoring ductility at the cost of strength. The annealing process involves recovery, recrystallization and sometimes grain growth, and depends on temperature and time.