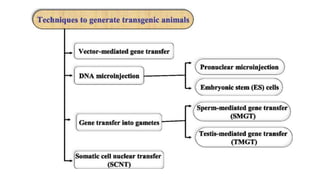
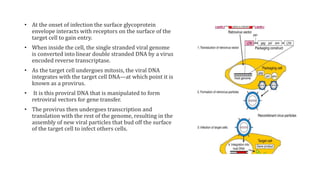
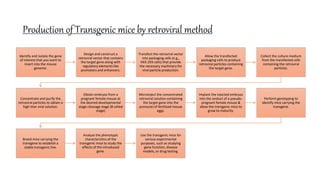
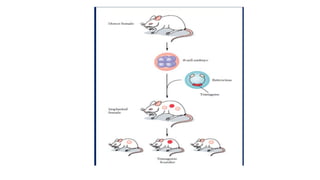
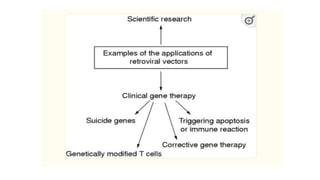
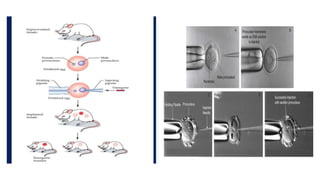
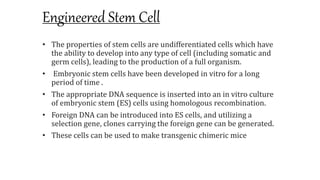
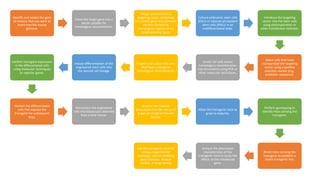
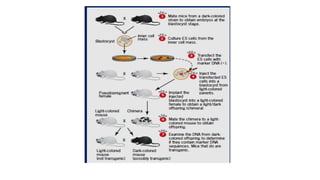
Methods for producing transgenic animals include retroviral, microinjection, and engineered stem cell methods. Transgenic animals can be identified through integration and expression methods like southern blot, PCR, dot blot, and protein expression analysis. The document discusses various transgenic animal production techniques in detail, including retroviral method, microinjection, and using engineered stem cells, outlining the key steps for each. It also covers transgene integration and identification methods.