This document discusses ANA profiles in connective tissue diseases. Some key points:
1. ANAs are autoantibodies that bind to nuclear structures and are seen at higher levels in patients with CTDs compared to normal individuals. Their detection is important for CTD diagnosis and treatment monitoring.
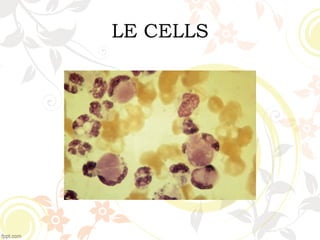
2. Historical studies first described CTDs like SLE and identified LE cells containing nuclear material, suggesting the presence of an anti-nuclear antibody.
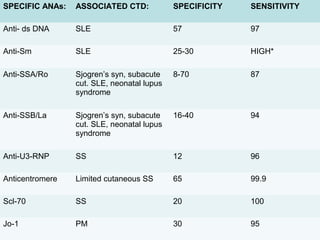
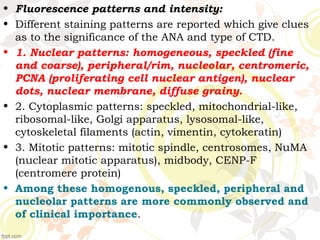
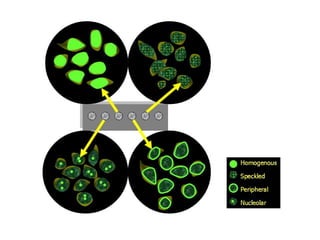
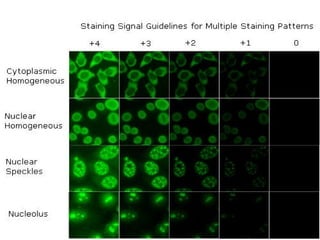
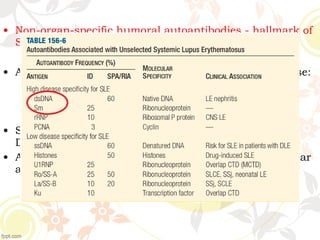
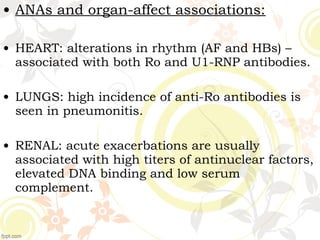
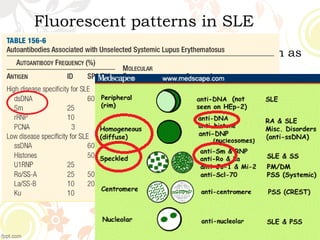
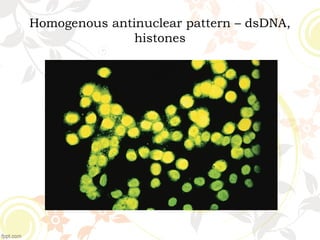
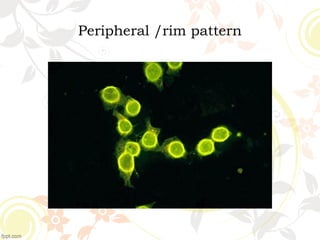
3. Common ANA patterns include homogeneous, speckled, peripheral and nucleolar and are associated with CTDs like SLE, Sjogren's syndrome and scleroderma. Specific ANAs like anti-dsDNA and anti-Sm are highly