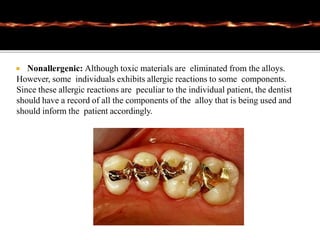
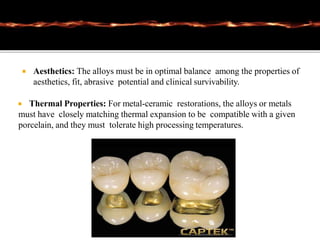
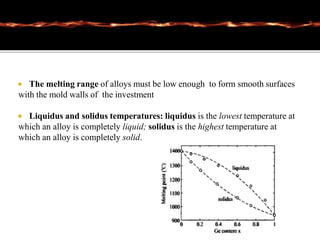
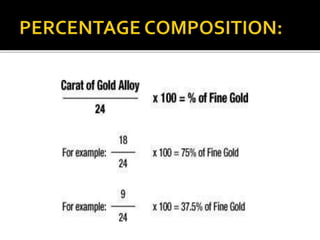
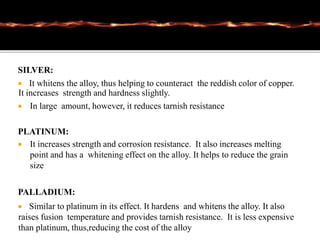
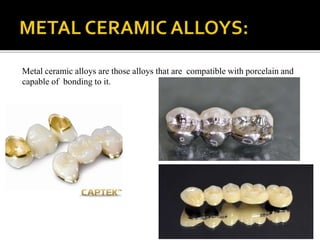
This document discusses casting alloys used in dentistry. It begins with a brief history of casting alloys and their evolution since the 1900s. It then covers the key properties casting alloys must have including biocompatibility, corrosion resistance, hardness, castability and bonding to ceramics. The document classifies casting alloys and discusses commonly used types such as gold alloys, silver-palladium alloys, cobalt-chrome alloys and titanium alloys. It provides details on the composition and characteristics of different alloy groups.