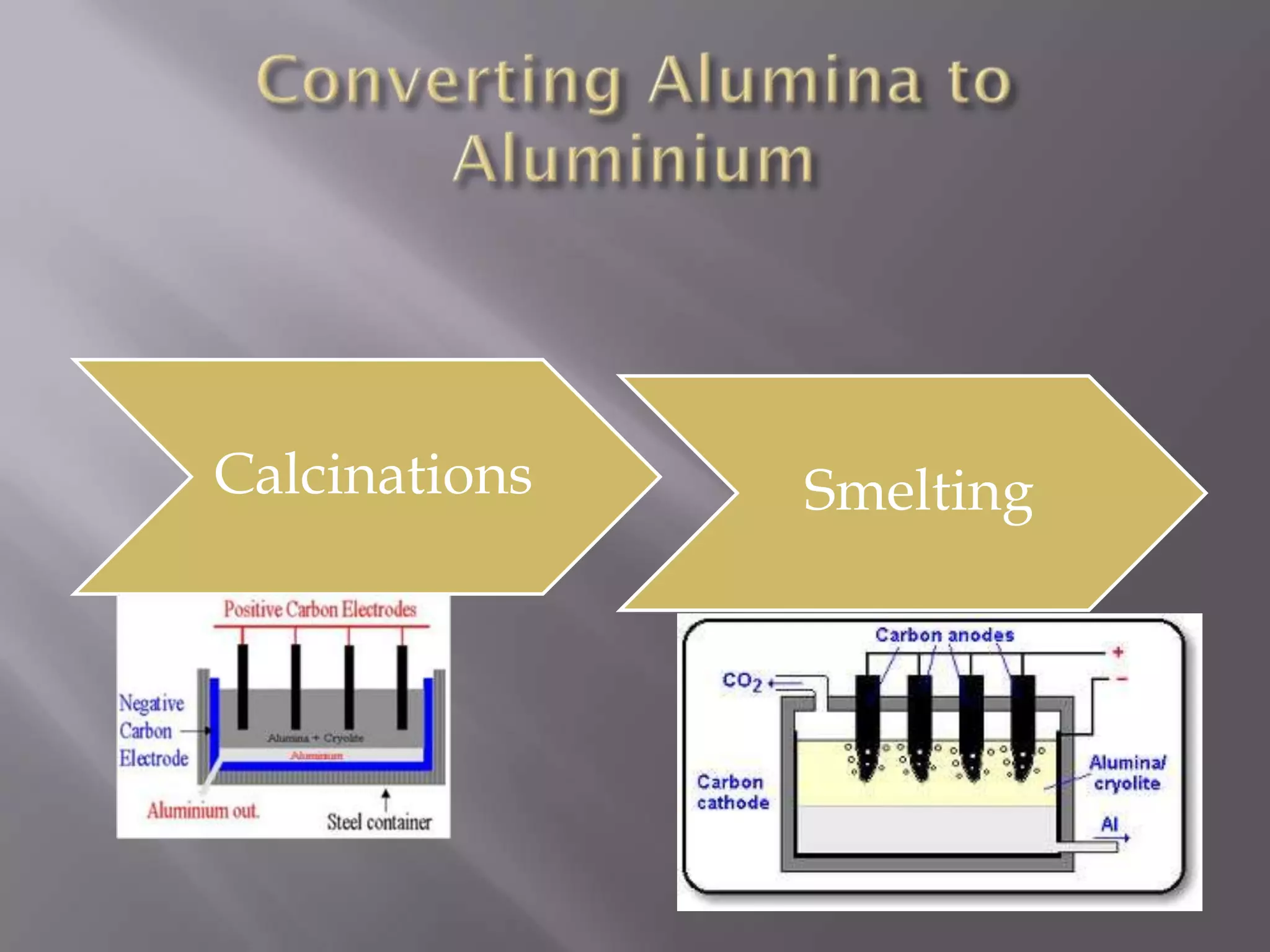
The document describes the process for extracting aluminum from bauxite ore. It involves crushing and grinding the bauxite, mixing it with a caustic soda solution in digesters, cooling the slurry, settling out the impurities, precipitating out the aluminum hydroxide, and calcining it to remove water. The aluminum hydroxide is then smelted using the Hall-Heroult process, which involves dissolving alumina in a molten cryolite bath using an electric current passing between carbon anodes and cathodes to produce molten aluminum.