Embed presentation
Downloaded 86 times
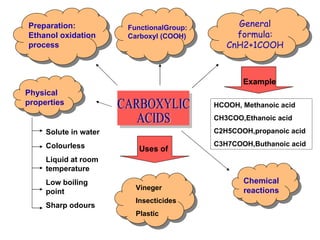


Carboxylic acids have the general formula CnH2+1COOH and are prepared through ethanol oxidation. They have a carboxyl functional group and are colorless liquids at room temperature with low boiling points and sharp odors. Common uses include vinegar, insecticides, and plastics. They undergo chemical reactions with bases to form salts and water, with carbonate metals to form salts, water and carbon dioxide, and with metals to form salts and hydrogen.

