The document describes various types of chemical reactions:
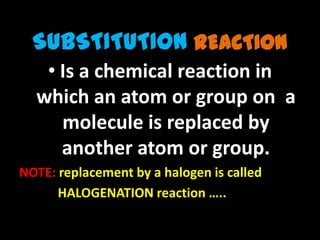
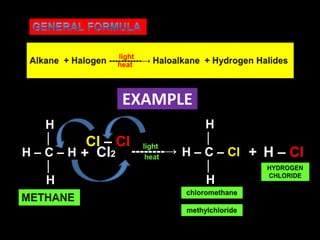
- Substitution reactions involve replacing an atom or group on a molecule with another atom or group. Halogenation is a substitution reaction that replaces an atom with a halogen.
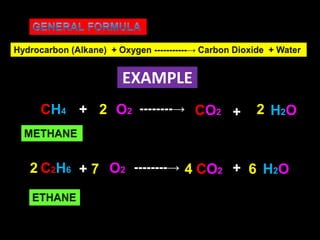
- Combustion reactions involve hydrocarbons reacting with oxygen to produce carbon dioxide and water, releasing a large amount of heat.
- Mechanics for a classroom activity are described where students will be divided into groups to perform reaction sequences within a time limit for a prize.