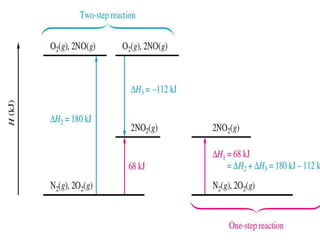
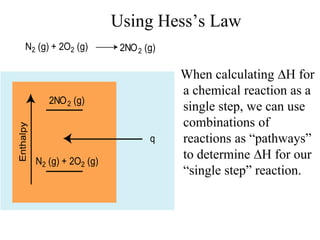
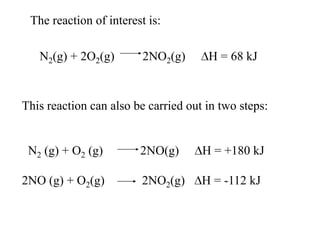
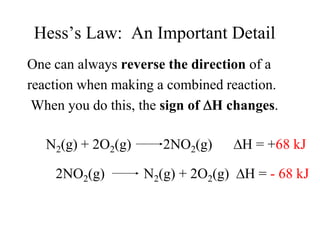
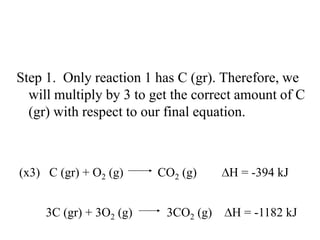
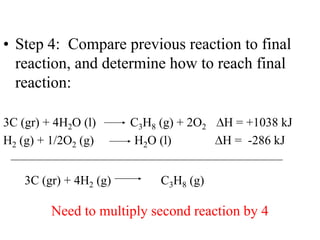
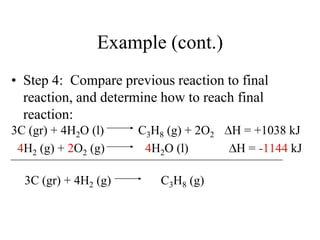
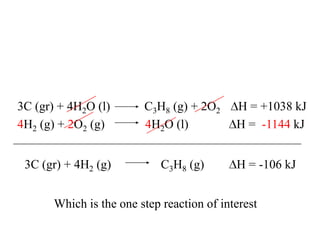
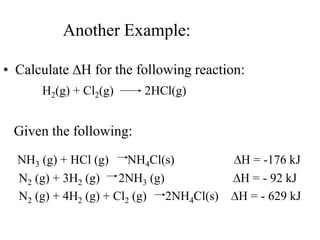
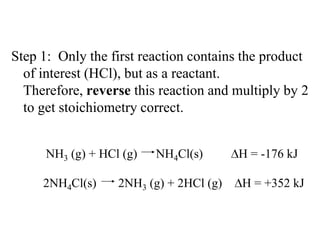
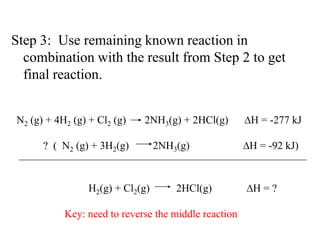
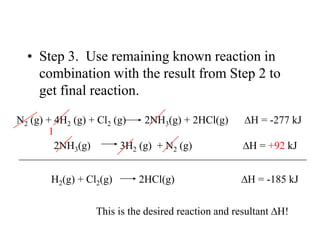
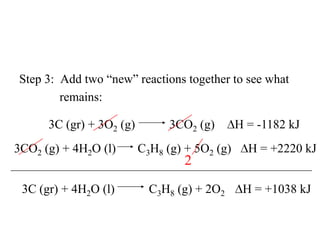Hess's law states that the enthalpy change of a reaction is independent of the pathway taken, as enthalpy is a state function. Using Hess's law, the enthalpy change of a reaction can be calculated by adding together enthalpy changes of multiple steps that combine to give the overall reaction. This is demonstrated with examples calculating the enthalpy change of reactions by combining known reaction enthalpies. Reactions can be reversed and coefficients adjusted as needed to achieve the desired overall reaction.