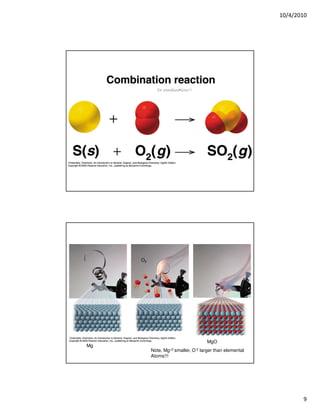
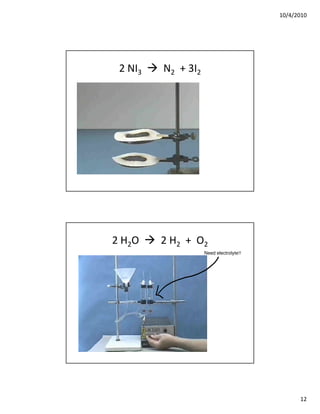
The document discusses three types of chemical reactions: combination reactions, combustion reactions, and decomposition reactions. It provides examples of each type of reaction and notes the key characteristics to look for in identifying them. Combination reactions involve two or more reactants forming one product. Combustion reactions involve a fuel reacting with oxygen to produce oxides. Decomposition reactions involve one reactant breaking down into two or more products.