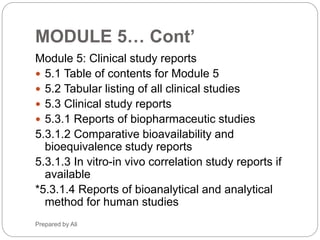
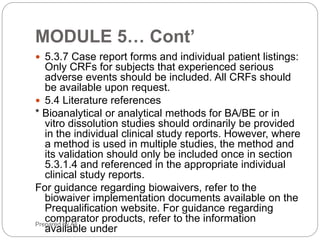
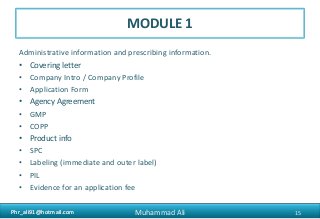
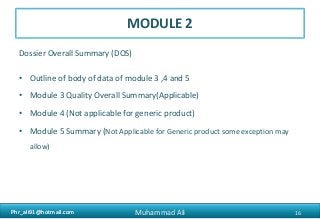
The document provides an overview of the Common Technical Document (CTD) format for organizing technical requirements for a generic drug product application to be submitted to regulatory authorities. It describes the CTD format, which includes Modules 1 through 5. Module 2 includes quality, non-clinical and clinical summaries. Module 3 contains information related to the drug substance and product quality. Module 5 contains clinical study reports, which for generic products would primarily include bioequivalence studies comparing the generic to an approved drug.