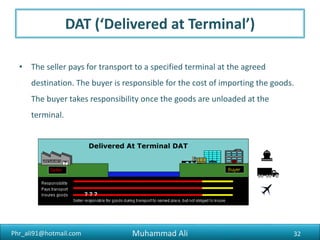
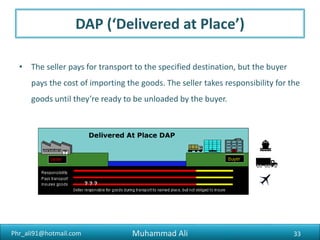
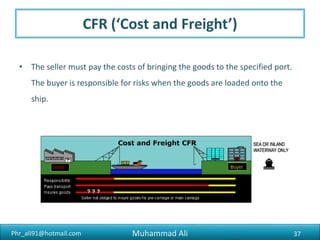
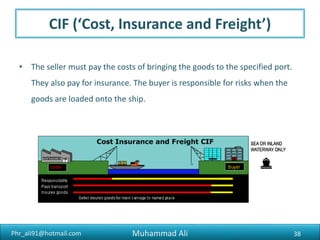
The document discusses the procedures, documentation requirements, and Incoterms for pharmaceutical exports. It covers the preliminary steps such as obtaining an IEC number, membership registrations, inquiries, order confirmations, financing, production, shipping, packing, quality control, and excise clearance. It also discusses export documentation requirements for banks, regulatory authorities, and buyers. Finally, it summarizes the key steps in an export transaction and classifies the various commercial and regulatory documents involved.