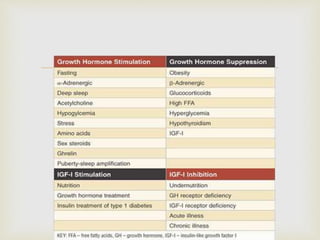
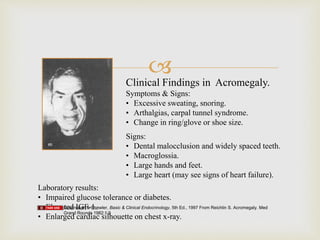
The document outlines clinical practice guidelines for diagnosing and managing acromegaly, emphasizing the importance of measuring IGF-1 levels in patients exhibiting related symptoms. It recommends transsphenoidal surgery as the primary treatment and details postoperative management, including monitoring hormone levels and imaging studies. Additional considerations are provided for comorbidities, medical therapy options, and special circumstances such as pregnancy.